|
Mineralizer
The purpose of a mineralizer is to facilitate the transport of insoluble “nutrient” to a seed crystal by means of a reversible chemical reaction. Over time, the seed crystal accumulates the material that was once in the nutrient and grows. Mineralizers are additives that aid the solubilization of the nutrient solid. When used in small quantities, mineralizers function as catalysts. Typically, a more stable solid is crystallized from a solution that consists of a less stable solid and a solvent. The process is done by dissolution-precipitation or crystallization process. Hydrothermal growth involves the crystallization of a dissolved solid at elevated temperatures. Often high pressures are involved. Historically, the goal of hydrothermal growth was to grow large crystals. Due to the recent developments in nanotechnology, small nanocrystals are now desired and made by hydrothermal growth with crystal size controlled by mineralizers. Different mineralizers result in crystals of diff ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Seed Crystal
A seed crystal is a small piece of single crystal or polycrystal material from which a large crystal of typically the same material is grown in a laboratory. Used to replicate material, the use of seed crystal to promote growth avoids the otherwise slow randomness of natural crystal growth and allows manufacture on a scale suitable for industry. Crystal enlargement The large crystal can be grown by dipping the seed into a supersaturated solution, into molten material that is then cooled, or by growth on the seed face by passing vapor of the material to be grown over it. Theory The theory behind this effect is thought to derive from the physical intermolecular interaction that occurs between compounds in a supersaturated solution (or possibly vapor). In solution, liberated (soluble) molecules (solute) are free to move about in random flow. This random flow permits for the possibility of two or more molecular compounds to interact. This interaction can potentiate intermolecular fo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Potassium Chloride
Potassium chloride (KCl, or potassium salt) is a metal halide salt composed of potassium and chlorine. It is odorless and has a white or colorless vitreous crystal appearance. The solid dissolves readily in water, and its solutions have a salt-like taste. Potassium chloride can be obtained from ancient dried lake deposits. KCl is used as a fertilizer, in medicine, in scientific applications, domestic water softeners (as a substitute for sodium chloride salt), and in food processing, where it may be known as E number additive E508. It occurs naturally as the mineral sylvite, and in combination with sodium chloride as sylvinite. Uses Fertilizer The majority of the potassium chloride produced is used for making fertilizer, called potash, since the growth of many plants is limited by potassium availability. Potassium chloride sold as fertilizer is known as muriate of potash (MOP). The vast majority of potash fertilizer worldwide is sold as MOP. Medical use Potassium is vital ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Zeolites
Zeolites are microporous, crystalline aluminosilicate materials commonly used as commercial adsorbents and catalysts. They mainly consist of silicon, aluminium, oxygen, and have the general formula ・y where is either a metal ion or H+. These positive ions can be exchanged for others in a contacting electrolyte solution. exchanged zeolites are particularly useful as solid acid catalysts. The term ''zeolite'' was originally coined in 1756 by Swedish mineralogist Axel Fredrik Cronstedt, who observed that rapidly heating a material, believed to have been stilbite, produced large amounts of steam from water that had been adsorbed by the material. Based on this, he called the material ''zeolite'', from the Greek , meaning "to boil" and , meaning "stone". Zeolites occur naturally but are also produced industrially on a large scale. , 253 unique zeolite frameworks have been identified, and over 40 naturally occurring zeolite frameworks are known. Every new zeolite structure that is obt ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Waters Of Hydration
In chemistry, water(s) of crystallization or water(s) of hydration are water molecules that are present inside crystals. Water is often incorporated in the formation of crystals from aqueous solutions. In some contexts, water of crystallization is the total mass of water in a substance at a given temperature and is mostly present in a definite (stoichiometric) ratio. Classically, "water of crystallization" refers to water that is found in the crystalline framework of a metal complex or a salt, which is not directly bonded to the metal cation. Upon crystallization from water, or water-containing solvents, many compounds incorporate water molecules in their crystalline frameworks. Water of crystallization can generally be removed by heating a sample but the crystalline properties are often lost. Compared to inorganic salts, proteins crystallize with large amounts of water in the crystal lattice. A water content of 50% is not uncommon for proteins. Applications Knowledge of hydr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
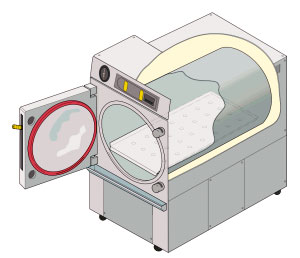
Autoclave
An autoclave is a machine used to carry out industrial and scientific processes requiring elevated temperature and pressure in relation to ambient pressure and/or temperature. Autoclaves are used before surgical procedures to perform sterilization and in the chemical industry to cure coatings and vulcanize rubber and for hydrothermal synthesis. Industrial autoclaves are used in industrial applications, especially in the manufacturing of composites. Many autoclaves are used to sterilize equipment and supplies by subjecting them to pressurized saturated steam at for around 30-60 minutes at a pressure of 15 psi (103 kPa or 1.02 atm) depending on the size of the load and the contents. The autoclave was invented by Charles Chamberland in 1879, although a precursor known as the steam digester was created by Denis Papin in 1679. The name comes from Greek ''auto-'', ultimately meaning self, and Latin ''clavis'' meaning key, thus a self-locking device. Uses Sterilization autoclav ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Silica
Silicon dioxide, also known as silica, is an oxide of silicon with the chemical formula , most commonly found in nature as quartz and in various living organisms. In many parts of the world, silica is the major constituent of sand. Silica is one of the most complex and most abundant families of materials, existing as a compound of several minerals and as a synthetic product. Notable examples include fused quartz, fumed silica, silica gel, opal and aerogels. It is used in structural materials, microelectronics (as an Insulator (electricity), electrical insulator), and as components in the food and pharmaceutical industries. Structure In the majority of silicates, the silicon atom shows tetrahedral coordination geometry, tetrahedral coordination, with four oxygen atoms surrounding a central Si atomsee 3-D Unit Cell. Thus, SiO2 forms 3-dimensional network solids in which each silicon atom is covalently bonded in a tetrahedral manner to 4 oxygen atoms. In contrast, CO2 is a linear ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Quartz
Quartz is a hard, crystalline mineral composed of silica (silicon dioxide). The atoms are linked in a continuous framework of SiO4 silicon-oxygen tetrahedra, with each oxygen being shared between two tetrahedra, giving an overall chemical formula of SiO2. Quartz is the second most abundant mineral in Earth's continental crust, behind feldspar. Quartz exists in two forms, the normal α-quartz and the high-temperature β-quartz, both of which are chiral. The transformation from α-quartz to β-quartz takes place abruptly at . Since the transformation is accompanied by a significant change in volume, it can easily induce microfracturing of ceramics or rocks passing through this temperature threshold. There are many different varieties of quartz, several of which are classified as gemstones. Since antiquity, varieties of quartz have been the most commonly used minerals in the making of jewelry and hardstone carvings, especially in Eurasia. Quartz is the mineral defining the val ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Zincite
Zincite is the mineral form of zinc oxide ( Zn O). Its crystal form is rare in nature; a notable exception to this is at the Franklin and Sterling Hill Mines in New Jersey, an area also famed for its many fluorescent minerals. It has a hexagonal crystal structure and a color that depends on the presence of impurities. The zincite found at the Franklin Furnace is red-colored, mostly due to iron and manganese dopants, and associated with willemite and franklinite. Zincite crystals can be grown artificially, and synthetic zincite crystals are available as a by-product of zinc smelting. Synthetic crystals can be colorless or can range in color from dark red, orange, or yellow to light green. Both natural and synthetic zincite crystals are significant for their early use as semiconductor crystal detectors in the early development of crystal radios before the advent of vacuum tubes. As an early radio detector it was used in conjunction with another mineral, galena, and this devic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sapphire
Sapphire is a precious gemstone, a variety of the mineral corundum, consisting of aluminium oxide () with trace amounts of elements such as iron, titanium, chromium, vanadium, or magnesium. The name sapphire is derived via the Latin "sapphirus" from the Greek "sappheiros", which referred to Lapis lazuli, lapis lazuli. It is typically blue, but natural "fancy" sapphires also occur in yellow, purple, orange, and green colors; "parti sapphires" show two or more colors. Red corundum stones also occur, but are called ruby, rubies rather than sapphires. Pink-colored corundum may be classified either as ruby or sapphire depending on locale. Commonly, natural sapphires are cut and polished into gemstones and worn in jewellery, jewelry. They also may be created synthetically in laboratories for industrial or decorative purposes in large boule (crystal), crystal boules. Because of the remarkable hardness of sapphires 9 on the Mohs scale of mineral hardness, Mohs scale (the third hardest ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lithium Chloride
Lithium chloride is a chemical compound with the formula Li Cl. The salt is a typical ionic compound (with certain covalent characteristics), although the small size of the Li+ ion gives rise to properties not seen for other alkali metal chlorides, such as extraordinary solubility in polar solvents (83.05 g/100 mL of water at 20 °C) and its hygroscopic properties. Chemical properties The salt forms crystalline hydrates, unlike the other alkali metal chlorides. Mono-, tri-, and pentahydrates are known. The anhydrous salt can be regenerated by heating the hydrates. LiCl also absorbs up to four equivalents of ammonia/mol. As with any other ionic chloride, solutions of lithium chloride can serve as a source of chloride ion, e.g., forming a precipitate upon treatment with silver nitrate: : LiCl + AgNO3 → AgCl + LiNO3 Preparation Lithium chloride is produced by treatment of lithium carbonate with hydrochloric acid. Anhydrous LiCl is prepared from the hydrate ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sodium Chloride
Sodium chloride , commonly known as salt (although sea salt also contains other chemical salts), is an ionic compound with the chemical formula NaCl, representing a 1:1 ratio of sodium and chloride ions. With molar masses of 22.99 and 35.45 g/mol respectively, 100 g of NaCl contains 39.34 g Na and 60.66 g Cl. Sodium chloride is the salt most responsible for the salinity of seawater and of the extracellular fluid of many multicellular organisms. In its edible form, salt (also known as ''table salt'') is commonly used as a condiment and food preservative. Large quantities of sodium chloride are used in many industrial processes, and it is a major source of sodium and chlorine compounds used as feedstocks for further chemical syntheses. Another major application of sodium chloride is de-icing of roadways in sub-freezing weather. Uses In addition to the familiar domestic uses of salt, more dominant applications of the approximately 250 million tonnes per year production (2008 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Reaction
A chemical reaction is a process that leads to the IUPAC nomenclature for organic transformations, chemical transformation of one set of chemical substances to another. Classically, chemical reactions encompass changes that only involve the positions of electrons in the forming and breaking of chemical bonds between atoms, with no change to the Atomic nucleus, nuclei (no change to the elements present), and can often be described by a chemical equation. Nuclear chemistry is a sub-discipline of chemistry that involves the chemical reactions of unstable and radioactive Chemical element, elements where both electronic and nuclear changes can occur. The substance (or substances) initially involved in a chemical reaction are called reagent, reactants or reagents. Chemical reactions are usually characterized by a chemical change, and they yield one or more Product (chemistry), products, which usually have properties different from the reactants. Reactions often consist of a sequence o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |