|
Michaelis–Becker Reaction
The Michaelis–Becker reaction is the reaction of a hydrogen phosphonate with a base, followed by a nucleophilic substitution of phosphorus on a haloalkane, to give an alkyl phosphonate. Yields of this reaction are often lower than the corresponding Michaelis–Arbuzov reaction The Michaelis–Arbuzov reaction (also called the Arbuzov reaction) is the chemical reaction of a trivalent phosphorus ester with an alkyl halide to form a pentavalent phosphorus species and another alkyl halide. The picture below shows the most c .... : Further reading * References {{DEFAULTSORT:Michaelis-Becker reaction Organic reactions Name reactions ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phosphonate
In organic chemistry, phosphonates or phosphonic acids are organophosphorus compounds containing groups (where R = alkyl, aryl, or just hydrogen). Phosphonic acids, typically handled as salts, are generally nonvolatile solids that are poorly soluble in organic solvents, but soluble in water and common alcohols. Many commercially important compounds are phosphonates, including glyphosate (the active molecule of the herbicide Roundup), and ethephon, a widely used plant growth regulator. Bisphosphonates are popular drugs for treatment of osteoporosis.Svara, J.; Weferling, N.; Hofmann, T. "Phosphorus Compounds, Organic," in ''Ullmann's Encyclopedia of Industrial Chemistry'', Wiley-VCH, Weinheim, 2008. . In biochemistry and medicinal chemistry, phosphonate groups are used as stable bioisoteres for phosphate, such as in the antiviral nucleotide analog, Tenofovir, one of the cornerstones of anti-HIV therapy. And there is an indication that phosphonate derivatives are "promising ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
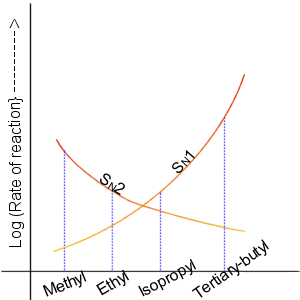
Nucleophilic Substitution
In chemistry, a nucleophilic substitution is a class of chemical reactions in which an electron-rich chemical species (known as a nucleophile) replaces a functional group within another electron-deficient molecule (known as the electrophile). The molecule that contains the electrophile and the leaving functional group is called the substrate. The most general form of the reaction may be given as the following: :\text\mathbf + \ce + \text\mathbf The electron pair (:) from the nucleophile (Nuc) attacks the substrate () and bonds with it. Simultaneously, the leaving group (LG) departs with an electron pair. The principal product in this case is . The nucleophile may be electrically neutral or negatively charged, whereas the substrate is typically neutral or positively charged. An example of nucleophilic substitution is the hydrolysis of an alkyl bromide, R-Br under basic conditions, where the attacking nucleophile is hydroxyl () and the leaving group is bromide (). :R-Br + OH- ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Haloalkane
The haloalkanes (also known as halogenoalkanes or alkyl halides) are alkanes containing one or more halogen substituents. They are a subset of the general class of halocarbons, although the distinction is not often made. Haloalkanes are widely used commercially. They are used as flame retardants, fire extinguishants, refrigerants, propellants, solvents, and pharmaceuticals. Subsequent to the widespread use in commerce, many halocarbons have also been shown to be serious pollutants and toxins. For example, the chlorofluorocarbons have been shown to lead to ozone depletion. Methyl bromide is a controversial fumigant. Only haloalkanes that contain chlorine, bromine, and iodine are a threat to the ozone layer, but fluorinated volatile haloalkanes in theory may have activity as greenhouse gases. Methyl iodide, a naturally occurring substance, however, does not have ozone-depleting properties and the United States Environmental Protection Agency has designated the compound a non-ozone l ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alkyl
In organic chemistry, an alkyl group is an alkane missing one hydrogen. The term ''alkyl'' is intentionally unspecific to include many possible substitutions. An acyclic alkyl has the general formula of . A cycloalkyl is derived from a cycloalkane by removal of a hydrogen atom from a Ring (chemistry), ring and has the general formula . Typically an alkyl is a part of a larger molecule. In structural formulae, the symbol R is used to designate a generic (unspecified) alkyl group. The smallest alkyl group is methyl, with the formula . Related concepts Alkylation is an important operation in refineries, for example in the production of high-octane gasoline. Alkylating antineoplastic agents are a class of compounds that are used to treat cancer. In such case, the term alkyl is used loosely. For example, nitrogen mustards are well-known alkylating agents, but they are not simple hydrocarbons. In chemistry, alkyl is a group, a substituent, that is attached to other molecular fr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Yield (chemistry)
In chemistry, yield, also referred to as reaction yield, is a measure of the quantity of Mole (unit), moles of a Product (chemistry), product formed in relation to the reactant consumed, obtained in a chemical reaction, usually expressed as a percentage. Yield is one of the primary factors that scientists must consider in organic synthesis, organic and inorganic chemical synthesis processes. In chemical reaction engineering, "yield", "Conversion (chemistry), conversion" and "selectivity" are terms used to describe ratios of how much of a reactant was consumed (conversion), how much desired product was formed (yield) in relation to the undesired product (selectivity), represented as X, Y, and S. Definitions In chemical reaction engineering, "yield", "Conversion (chemistry), conversion" and "selectivity" are terms used to describe ratios of how much of a reactant has reacted—conversion, how much of a desired product was formed—yield, and how much desired product was formed ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Michaelis–Arbuzov Reaction
The Michaelis–Arbuzov reaction (also called the Arbuzov reaction) is the chemical reaction of a trivalent phosphorus ester with an alkyl halide to form a pentavalent phosphorus species and another alkyl halide. The picture below shows the most common types of substrates undergoing the Arbuzov reaction; phosphite esters (1) react to form phosphonates (2), phosphonites (3) react to form phosphinates (4) and phosphinites (5) react to form phosphine oxides (6). The reaction was discovered by August Michaelis in 1898, and greatly explored by Aleksandr Arbuzov soon thereafter. This reaction is widely used for the synthesis of various phosphonates, phosphinates, and phosphine oxides. Several reviews have been published. The reaction also occurs for coordinated phosphite ligands, as illustrated by the demethylation of 2+ to give −, which is called the Klaui ligand. Reaction mechanism file:Michaelis-Arbuzov Reaction Mechanism.png, center, 600px, The mechanism of the Michaelis–Arbu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organic Reactions
Organic reactions are chemical reactions involving organic compounds. The basic organic chemistry reaction types are addition reactions, elimination reactions, substitution reactions, pericyclic reactions, rearrangement reactions, photochemical reactions and redox reactions. In organic synthesis, organic reactions are used in the construction of new organic molecules. The production of many man-made chemicals such as drugs, plastics, food additives, fabrics depend on organic reactions. The oldest organic reactions are combustion of organic fuels and saponification of fats to make soap. Modern organic chemistry starts with the Wöhler synthesis in 1828. In the history of the Nobel Prize in Chemistry awards have been given for the invention of specific organic reactions such as the Grignard reaction in 1912, the Diels-Alder reaction in 1950, the Wittig reaction in 1979 and olefin metathesis in 2005. Classifications Organic chemistry has a strong tradition of naming a specific reac ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |