|
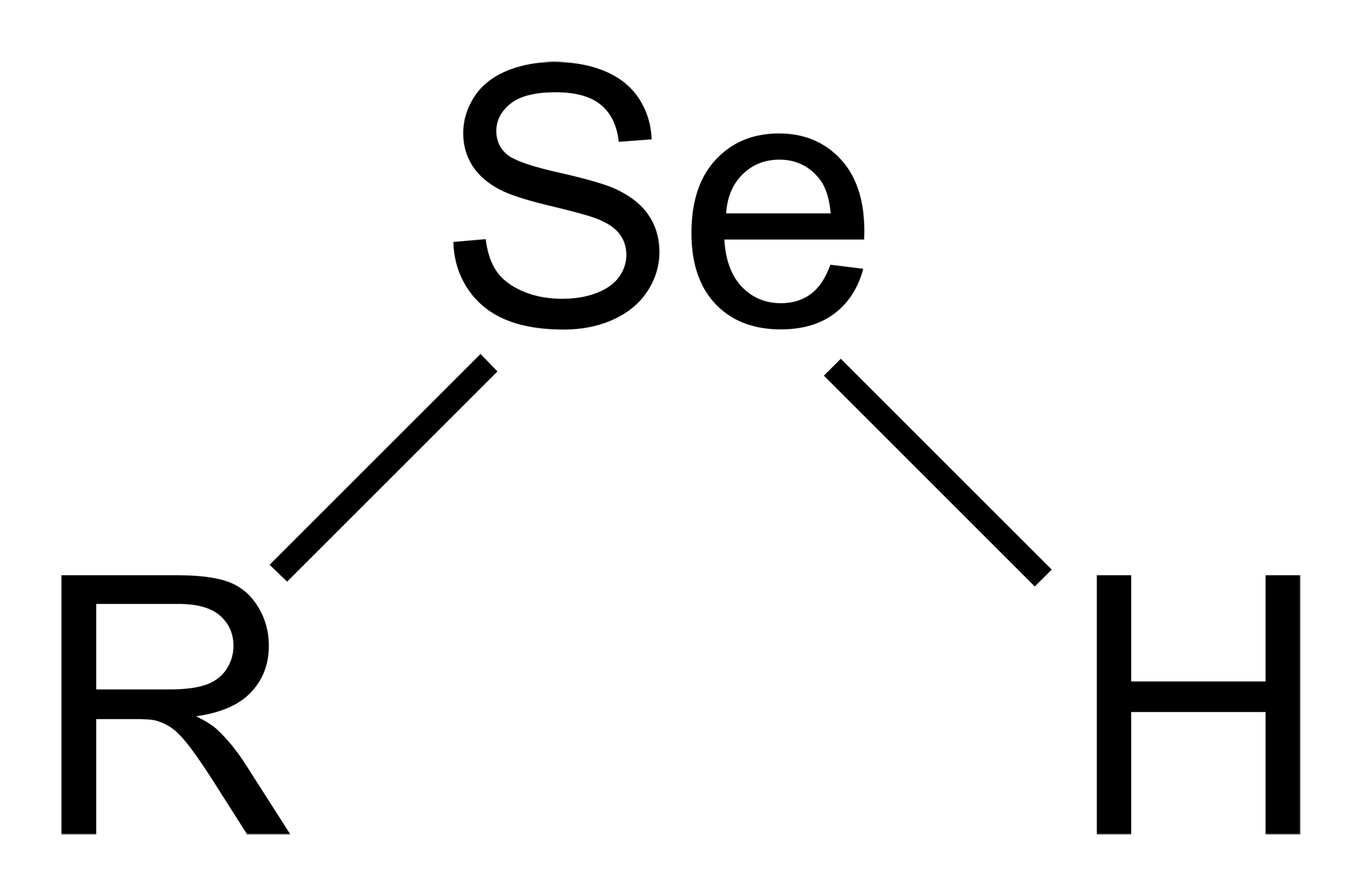
Methylselenol
Selenols are organic compounds that contain the functional group with the connectivity C– Se–H. Selenols are sometimes also called selenomercaptans and selenothiols. Selenols are one of the principal classes of organoselenium compounds. The best known member of the group is the amino acid selenocysteine. Structure, bonding, properties Selenols are structurally similar to thiols, but the C-Se bond is about 8% longer at 196 pm. The C–Se–H angle approaches 90°. The bonding involves almost pure p-orbitals on Se, hence the near 90 angles. The Se–H bond energy is weaker than the S–H bond, consequently selenols are easily oxidized and serve as H-atom donors. The Se-H bond is much weaker than the S-H bond as reflected in their respective bond dissociation energy (BDE). For C6H5Se-H, the BDE is 326 kJ/mol, while for C6H5S-H, the BDE is 368 kJ/mol. Selenol acids are about 1000 times stronger than thiols: the p''K''a of CH3SeH is 5.2 vs 8.3 for CH3SH. Deprotonation affords the s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Essential Amino Acid
An essential amino acid, or indispensable amino acid, is an amino acid that cannot be synthesized from scratch by the organism fast enough to supply its demand, and must therefore come from the diet. Of the 21 amino acids common to all life forms, the nine amino acids humans cannot synthesize are phenylalanine, valine, threonine, tryptophan, methionine, leucine, isoleucine, lysine, and histidine. Six other amino acids are considered conditionally essential in the human diet, meaning their synthesis can be limited under special pathophysiological conditions, such as prematurity in the infant or individuals in severe catabolic distress. These six are arginine, cysteine, glycine, glutamine, proline, and tyrosine. Six amino acids are non-essential (dispensable) in humans, meaning they can be synthesized in sufficient quantities in the body. These six are alanine, aspartic acid, asparagine, glutamic acid, serine, and selenocysteine (considered the 21st amino acid). Pyrrolysine (consi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Metallomics (journal)
''Metallomics'' is a monthly peer-reviewed scientific journal covering the growing research field of metallomics. The journal's scope is aimed at "elucidating the identification, distribution, dynamics, role and impact of metals and metalloids in biological systems". It is published by the Royal Society of Chemistry. The executive editor is Jeanne Andres, while the current chair of the editorial board is David Giedroc at Indiana University Bloomington. Abstracting and indexing The journal is abstracted and indexed in: * Chemical Abstracts Service/CASSI * PubMed/MEDLINE * Science Citation Index * Scopus According to the ''Journal Citation Reports'', the journal has a 2020 impact factor The impact factor (IF) or journal impact factor (JIF) of an academic journal is a scientometric index calculated by Clarivate that reflects the yearly mean number of citations of articles published in the last two years in a given journal, as i ... of 4.526. References External links * ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Selenourea
Selenourea is the organoselenium compound with the formula SeC(NH2)2. It is a white solid. This compound features a rare example of a stable, unhindered carbon-selenium double bond. The compound is used in the synthesis of selenium heterocycles. Compared to urea, the oxo-analog of selenourea, few studies have been done on the compound due to the instability and toxicity of selenium compounds. Selenourea is toxic if inhaled or consumed. Synthesis The compound was first synthesized in 1884 by Auguste Verneuil by the reaction of hydrogen selenide and cyanamide: :H2Se + NCNH2 → SeC(NH2)2 While this reaction has even found use in industrial synthesis of selenourea, more modern methods concern themselves with synthesis of substituted selenoureas. These can be synthesized using organic isoselenocyanates and secondary amines: :RN=C=Se + NHR′R″ → Se=C(NRH)(NR′R″H) Alternatively, a substituted carbodiimide could be used as follows: :RN=C=NR′ Se=C(NRH)(NR′H) Prope ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alkylation
Alkylation is the transfer of an alkyl group from one molecule to another. The alkyl group may be transferred as an alkyl carbocation, a free radical, a carbanion, or a carbene (or their equivalents). Alkylating agents are reagents for effecting alkylation. Alkyl groups can also be removed in a process known as dealkylation. Alkylating agents are often classified according to their nucleophilic or electrophilic character. In oil refining contexts, alkylation refers to a particular alkylation of isobutane with olefins. For upgrading of petroleum, alkylation produces a premium blending stock for gasoline. In medicine, alkylation of DNA is used in chemotherapy to damage the DNA of cancer cells. Alkylation is accomplished with the class of drugs called alkylating antineoplastic agents. Nucleophilic alkylating agents Nucleophilic alkylating agents deliver the equivalent of an alkyl anion ( carbanion). The formal "alkyl anion" attacks an electrophile, forming a new covalent ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Selenophenol
Benzeneselenol, also known as selenophenol, is the organoselenium compound with the formula C6H5SeH, often abbreviated PhSeH. It is the selenium analog of phenol. This colourless, malodorous compound is a reagent in organic synthesis. Synthesis Benzeneselenol is prepared by the reaction of phenylmagnesium bromide and selenium: :PhMgBr + Se → PhSeMgBr :PhSeMgBr + HCl → PhSeH + MgBrCl Since benzeneselenol does not have a long shelflife, it is often generated in situ. A common method is by reduction of diphenyldiselenide. A further reason for this conversion is that often, it is the anion that is sought. Reactions More so than thiophenol, benzeneselenol is easily oxidized by air. The facility of this reaction reflects the weakness of the Se-H bond, bond dissociation energy of which is estimated to be between 67 and 74 kcal/mol. In contrast, the S-H BDE for thiophenol is near 80 kcal/mol. The product is diphenyl diselenide as shown in this idealized equation: : ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organic Syntheses
''Organic Syntheses'' is a peer-reviewed scientific journal that was established in 1921. It publishes detailed and checked procedures for the synthesis of organic compounds. A unique feature of the review process is that all of the data and experiments reported in an article must be successfully repeated in the laboratory of a member of the editorial board as a check for reproducibility prior to publication. The journal is published by Organic Syntheses, Inc., a non-profit corporation. An annual print version is published by John Wiley & Sons on behalf of Organic Syntheses, Inc. History Prior to World War I, work on synthetic organic chemistry in the United States had been quite limited, and most of the reagents used in laboratories had to be imported from Europe. When export stoppages and trade embargoes cut off this source, Clarence Derick, a professor of chemistry at University of Illinois at Urbana-Champaign, began an effort to synthesize these needed chemicals in industr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phenylmagnesium Bromide
Phenylmagnesium bromide, with the simplified formula , is a magnesium-containing organometallic compound. It is commercially available as a solution in diethyl ether or tetrahydrofuran (THF). Phenylmagnesium bromide is a Grignard reagent. It is often used as a synthetic equivalent for the phenyl "Ph−" synthon. Preparation Phenylmagnesium bromide is commercially available as solutions of diethyl ether or THF. Laboratory preparation involves treating bromobenzene with magnesium metal, usually in the form of turnings. A small amount of iodine may be used to activate the magnesium to initiate the reaction. Coordinating solvents such as ether or THF, are required to solvate (complex) the magnesium(II) center. The solvent must be aprotic since alcohols and water contain an acidic proton and thus react with phenylmagnesium bromide to give benzene. Carbonyl-containing solvents, such as acetone and ethyl acetate, are also incompatible with the reagent. Structure Although phenylmagn ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Benzeneselenol
Benzeneselenol, also known as selenophenol, is the organoselenium compound with the formula C6H5SeH, often abbreviated PhSeH. It is the selenium analog of phenol. This colourless, malodorous compound is a reagent in organic synthesis. Synthesis Benzeneselenol is prepared by the reaction of phenylmagnesium bromide and selenium: :PhMgBr + Se → PhSeMgBr :PhSeMgBr + HCl → PhSeH + MgBrCl Since benzeneselenol does not have a long shelflife, it is often generated in situ. A common method is by reduction of diphenyldiselenide. A further reason for this conversion is that often, it is the anion that is sought. Reactions More so than thiophenol, benzeneselenol is easily oxidized by air. The facility of this reaction reflects the weakness of the Se-H bond, bond dissociation energy of which is estimated to be between 67 and 74 kcal/mol. In contrast, the S-H BDE for thiophenol is near 80 kcal/mol. The product is diphenyl diselenide as shown in this idealized equation: : ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Grignard Reagent
A Grignard reagent or Grignard compound is a chemical compound with the general formula , where X is a halogen and R is an organic group, normally an alkyl or aryl. Two typical examples are methylmagnesium chloride and phenylmagnesium bromide . They are a subclass of the organomagnesium compounds. Grignard compounds are popular reagents in organic synthesis for creating new carbon-carbon bonds. For example, when reacted with another halogenated compound in the presence of a suitable catalyst, they typically yield and the magnesium halide as a byproduct; and the latter is insoluble in the solvents normally used. In this aspect, they are similar to organolithium reagents. Pure Grignard reagents are extremely reactive solids. They are normally handled as solutions in solvents such as diethyl ether or tetrahydrofuran; which are relatively stable as long as water is excluded. In such a medium, a Grignard reagent is invariably present as a complex with the magnesium atom conn ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organolithium Reagent
In organometallic chemistry, organolithium reagents are chemical compounds that contain carbon–lithium (C–Li) bonds. These reagents are important in organic synthesis, and are frequently used to transfer the organic group or the lithium atom to the substrates in synthetic steps, through nucleophilic addition or simple deprotonation. Organolithium reagents are used in industry as an initiator for anionic polymerization, which leads to the production of various elastomers. They have also been applied in asymmetric synthesis in the pharmaceutical industry. Due to the large difference in electronegativity between the carbon atom and the lithium atom, the C−Li bond is highly ionic. Owing to the polar nature of the C−Li bond, organolithium reagents are good nucleophiles and strong bases. For laboratory organic synthesis, many organolithium reagents are commercially available in solution form. These reagents are highly reactive, and are sometimes pyrophoric. History and dev ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Research In Toxicology
''Chemical Research in Toxicology'' is a peer-reviewed scientific journal, published since 1988 by the American Chemical Society. It is currently abstracted and indexed in Chemical Abstracts Service, Scopus, EBSCOhost, PubMed, CABI, Science Citation Index Expanded, and SwetsWise. As of January 2018, the editor-in-chief is Shana Sturla (Institute of Food, Nutrition, and Health Department of Health Sciences and Technology ETH Zurich).. According to the ''Journal Citation Reports'', the journal had a 2020 impact factor The impact factor (IF) or journal impact factor (JIF) of an academic journal is a scientometric index calculated by Clarivate that reflects the yearly mean number of citations of articles published in the last two years in a given journal, as i ... of 3.739. References External links * {{DEFAULTSORT:Chemical Research In Toxicology American Chemical Society academic journals Toxicology journals Publications established in 1988 Monthly journals English ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |