|
Methylcitrate Cycle
The methylcitrate cycle, or the MCC, is the mechanism by which propionyl-CoA is formed, generated by β-oxidation of odd-chain fatty acids, and broken down to its final products, succinate and pyruvate. The methylcitrate cycle is closely related to both the citric acid cycle and the glyoxylate cycle, in that they share substrates, enzymes and products. The methylcitrate cycle functions overall to detoxify bacteria of toxic propionyl-CoA, and plays an essential role in propionate metabolism in bacteria. Incomplete propionyl-CoA metabolism may lead to the buildup of toxic metabolites in bacteria, and thus the function of the methylcitrate cycle is an important biological process. History 2-methyl''iso''citric acid, an intermediate of the methylcitrate cycle, was first synthesized in 1886 as a mixture of four isomers. The pathway of the methylcitrate cycle was not discovered until 1973 in fungi, though it was not yet fully understood. Originally, the methylcitrate cycle was thoug ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
MethylCitrate Cycle
The methylcitrate cycle, or the MCC, is the mechanism by which propionyl-CoA is formed, generated by β-oxidation of odd-chain fatty acids, and broken down to its final products, succinate and pyruvate. The methylcitrate cycle is closely related to both the citric acid cycle and the glyoxylate cycle, in that they share substrates, enzymes and products. The methylcitrate cycle functions overall to detoxify bacteria of toxic propionyl-CoA, and plays an essential role in propionate metabolism in bacteria. Incomplete propionyl-CoA metabolism may lead to the buildup of toxic metabolites in bacteria, and thus the function of the methylcitrate cycle is an important biological process. History 2-methyl''iso''citric acid, an intermediate of the methylcitrate cycle, was first synthesized in 1886 as a mixture of four isomers. The pathway of the methylcitrate cycle was not discovered until 1973 in fungi, though it was not yet fully understood. Originally, the methylcitrate cycle was thoug ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Propionyl-CoA Synthase
Propionyl-CoA is a coenzyme A derivative of propionic acid. It is composed of a 24 total carbon chain (without the coenzyme, it is a 3 carbon structure) and its production and metabolic fate depend on which organism it is present in. Several different pathways can lead to its production, such as through the catabolism of specific amino acids or the oxidation of odd-chain fatty acids. It later can be broken down by propionyl-CoA carboxylase or through the methylcitrate cycle. In different organisms, however, propionyl-CoA can be sequestered into controlled regions, to alleviate its potential toxicity through accumulation. Genetic deficiencies regarding the production and breakdown of propionyl-CoA also have great clinical and human significance. Production There are several different pathways through which propionyl-CoA can be produced: * Propionyl-CoA, a three-carbon structure, is considered to be a minor species of propionic acid. Therefore, odd-number chains of fatty acid ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Propionate
Propionic acid (, from the Greek words πρῶτος : ''prōtos'', meaning "first", and πίων : ''píōn'', meaning "fat"; also known as propanoic acid) is a naturally occurring carboxylic acid with chemical formula CH3CH2CO2H. It is a liquid with a pungent and unpleasant smell somewhat resembling body odor. The anion CH3CH2CO2− as well as the salts and esters of propionic acid are known as propionates or propanoates. History Propionic acid was first described in 1844 by Johann Gottlieb, who found it among the degradation products of sugar. Over the next few years, other chemists produced propionic acid by different means, none of them realizing they were producing the same substance. In 1847, French chemist Jean-Baptiste Dumas established all the acids to be the same compound, which he called propionic acid, from the Greek words πρῶτος (prōtos), meaning ''first'', and πίων (piōn), meaning ''fat'', because it is the smallest H(CH2)''n''COOH acid that exhibit ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acetate
An acetate is a salt (chemistry), salt formed by the combination of acetic acid with a base (e.g. Alkali metal, alkaline, Alkaline earth metal, earthy, Transition metal, metallic, nonmetallic or radical Radical (chemistry), base). "Acetate" also describes the conjugate acid, conjugate base or ion (specifically, the negatively charged ion called an anion) typically found in aqueous solution and written with the chemical formula . The neutral molecules formed by the combination of the acetate ion and a ''positive'' ion (called a cation) are also commonly called "acetates" (hence, ''acetate of lead'', ''acetate of aluminum'', etc.). The simplest of these is hydrogen acetate (called acetic acid) with corresponding salts, esters, and the polyatomic ion, polyatomic anion , or . Most of the approximately 5 billion kilograms of acetic acid produced annually in industry are used in the production of acetates, which usually take the form of polymers. In nature, acetate is the most common ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bond Cleavage
In chemistry, bond cleavage, or bond fission, is the splitting of chemical bonds. This can be generally referred to as dissociation when a molecule is cleaved into two or more fragments. In general, there are two classifications for bond cleavage: ''homo''lytic and ''hetero''lytic, depending on the nature of the process. The triplet and singlet excitation energies of a sigma bond can be used to determine if a bond will follow the homolytic or heterolytic pathway. A metal−metal sigma bond is an exception because the bond's excitation energy is extremely high, thus cannot be used for observation purposes. In some cases, bond cleavage requires catalysts. Due to the high bond-dissociation energy of C-H bonds, around , a large amount of energy is required to cleave the hydrogen atom from the carbon and bond a different atom to the carbon. Homolytic cleavage In homolytic cleavage, or homolysis, the two electrons in a cleaved covalent bond are divided equally between th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Methylisocitrate Lyase
The enzyme methylisocitrate lyase () catalyzes the chemical reaction :(2''S'',3''R'')-3-hydroxybutane-1,2,3-tricarboxylate \rightleftharpoons pyruvate + succinate The reaction is similar to that of isocitrate lyase, except that an additional methyl group (marked with an asterisk in the above scheme) is present, meaning that citrate is replaced by methylcitrate and glyoxylate by pyruvate. In fact, in some bacteria such as ''Mycobacterium tuberculosis'', isocitrate lyase actually plays the role of methylisocitrate lyase. This enzyme belongs to the family of lyases, specifically the oxo-acid-lyases, which cleave carbon-carbon bonds. The systematic name of this enzyme class is (2S,3R)-3-hydroxybutane-1,2,3-tricarboxylate pyruvate-lyase (succinate-forming). Other names in common use include 2-methylisocitrate lyase, MICL, and (2S,3R)-3-hydroxybutane-1,2,3-tricarboxylate pyruvate-lyase. This enzyme participates in propanoate metabolism. Methylisocitrate lyase was discovered in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Succinate
Succinic acid () is a dicarboxylic acid with the chemical formula (CH2)2(CO2H)2. The name derives from Latin ''succinum'', meaning amber. In living organisms, succinic acid takes the form of an anion, succinate, which has multiple biological roles as a metabolic intermediate being converted into fumarate by the enzyme succinate dehydrogenase in complex 2 of the electron transport chain which is involved in making ATP, and as a signaling molecule reflecting the cellular metabolic state. It is marketed as food additive E363. Succinate is generated in mitochondria via the tricarboxylic acid cycle (TCA). Succinate can exit the mitochondrial matrix and function in the cytoplasm as well as the extracellular space, changing gene expression patterns, modulating epigenetic landscape or demonstrating hormone-like signaling. As such, succinate links cellular metabolism, especially ATP formation, to the regulation of cellular function. Dysregulation of succinate synthesis, and therefore A ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isomerization
In chemistry, isomerization or isomerisation is the process in which a molecule, polyatomic ion or molecular fragment is transformed into an isomer with a different chemical structure. Enolization is an example of isomerization, as is tautomerization. When the isomerization occurs intramolecularly it may be called a rearrangement reaction. When the activation energy for the isomerization reaction is sufficiently small, both isomers will exist in a temperature-dependent equilibrium with each other. Many values of the standard free energy difference, \Delta G^\circ, have been calculated, with good agreement between observed and calculated data. Examples and applications Alkanes Skeletal isomerization occurs in the cracking process, used in the petrochemical industry. As well as reducing the average chain length, straight-chain hydrocarbons are converted to branched isomers in the process, as illustrated the following reaction of ''n''-butane to ''i''-butane. :\overset -> \o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
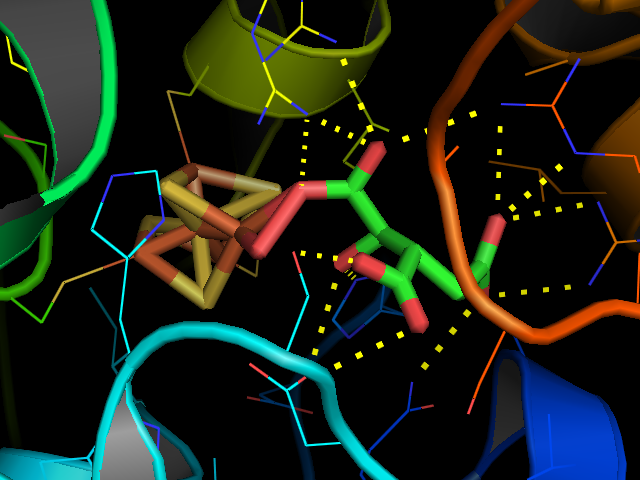
Aconitase
Aconitase (aconitate hydratase; ) is an enzyme that catalyses the stereo-specific isomerization of citrate to isocitrate via ''cis''- aconitate in the tricarboxylic acid cycle, a non-redox-active process. Image:Citrate wpmp.png, Image:Cis-Aconitate wpmp.png, Image:isocitric acid.svg, Structure Aconitase, displayed in the structures in the right margin of this page, has two slightly different structures, depending on whether it is activated or inactivated. In the inactive form, its structure is divided into four domains. Counting from the N-terminus, only the first three of these domains are involved in close interactions with the Fe-4Scluster, but the active site consists of residues from all four domains, including the larger C-terminal domain. The Fe-S cluster and a anion also reside in the active site. When the enzyme is activated, it gains an additional iron atom, creating a Fe-4Scluster. However, the structure of the rest of the enzyme is nearly unchanged; the c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |