|
Methylarsonic Acid
Methylarsonic acid is an organoarsenic compound with the formula CH3AsO3H2. It is a colorless, water-soluble solid. Salts of this compound, e.g. disodium methyl arsonate, have been widely used in as herbicides and fungicides in growing cotton and rice. Reactions Near physiological pH, methanearsonic acid converts to its conjugate bases, the methylarsonates. These include CH3AsO3H− and . Synthesis and biosynthesis Reaction of arsenous acid with methyl iodide gives methylarsonic acid. This historically significant conversion is called the Meyer reaction: :As(OH)3 + CH3I + NaOH → CH3AsO(OH)2 + NaI + H2O The then-novel aspect of the reaction was that alkylation occurs at arsenic, leading to oxidation of arsenic from oxidation state +3 to +5. The biomethylation of arsenic compounds is thought to start with the formation of methanearsonates. Thus, trivalent arsenic compounds are methylated to give methanearsonate. S-Adenosylmethionine, ''S''-Adenosylmethionine is the met ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
International Union Of Pure And Applied Chemistry
The International Union of Pure and Applied Chemistry (IUPAC ) is an international federation of National Adhering Organizations working for the advancement of the chemical sciences, especially by developing nomenclature and terminology. It is a member of the International Science Council (ISC). IUPAC is registered in Zürich, Switzerland, and the administrative office, known as the "IUPAC Secretariat", is in Research Triangle Park, North Carolina, United States. This administrative office is headed by IUPAC's executive director, currently Lynn Soby. IUPAC was established in 1919 as the successor of the International Congress of Applied Chemistry for the advancement of chemistry. Its members, the National Adhering Organizations, can be national chemistry societies, national academies of sciences, or other bodies representing chemists. There are fifty-four National Adhering Organizations and three Associate National Adhering Organizations. IUPAC's Inter-divisional Committee on ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Royal Society Of Chemistry
The Royal Society of Chemistry (RSC) is a learned society (professional association) in the United Kingdom with the goal of "advancing the chemistry, chemical sciences". It was formed in 1980 from the amalgamation of the Chemical Society, the Royal Institute of Chemistry, the Faraday Society, and the Society for Analytical Chemistry with a new Royal Charter and the dual role of learned society and professional body. At its inception, the Society had a combined membership of 34,000 in the UK and a further 8,000 abroad. The headquarters of the Society are at Burlington House, Piccadilly, London. It also has offices in Thomas Graham House in Cambridge (named after Thomas Graham (chemist), Thomas Graham, the first president of the Chemical Society) where ''RSC Publishing'' is based. The Society has offices in the United States, on the campuses of The University of Pennsylvania and Drexel University, at the University City Science Center in Philadelphia, Pennsylvania, in both Beijing a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organoarsenic Compound
Organoarsenic chemistry is the chemistry of compounds containing a chemical bond between arsenic and carbon. A few organoarsenic compounds, also called "organoarsenicals," are produced industrially with uses as insecticides, herbicides, and fungicides. In general these applications are declining in step with growing concerns about their impact on the environment and human health. The parent compounds are arsane and arsenic acid. Despite their toxicity, organoarsenic biomolecules are well known. History 140px, Cacodyl (tetramethyldiarsine) was one of the first organoarsenic compounds. Surprising for an area now considered of minor importance, organoarsenic chemistry played a prominent role in the history of the field of chemistry. The oldest known organoarsenic compound, the foul smelling cacodyl was reported in "cacodyl" (1760) and is sometimes classified as the first synthetic organometallic compound. The compound Salvarsan was one of the first pharmaceuticals, earning a Nobel ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Disodium Methyl Arsonate
Disodium methyl arsonate (DSMA) is the organoarsenic compound with the formula CH3AsO3Na2. It is a colorless, water-soluble solid derived from methanearsonic acid. It is used as a herbicide. Tradenames include Metharsinat, Arrhenal, Disomear, Metharsan, Stenosine, Tonarsan, Tonarsin, Arsinyl, Arsynal, and Diarsen. The EPA states that all forms of arsenic are a serious risk to human health and the United States' Agency for Toxic Substances and Disease Registry ranked arsenic as number 1 in its 2001 Priority List of Hazardous Substances at Superfund sites. Arsenic is classified as a Group-A carcinogen. The EPA states that: The EPA also states that, while contaminated soil poses a serious risk to health, arsenic frequently mobilizes from soils and other sources, ending up in water where it is even more of a toxicity issue. See also * Cacodylic acid * Monosodium methyl arsenate Monosodium methyl arsenate (MSMA) is an arsenic-based herbicide. It is an organo-arsenate; less t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Arsenous Acid
Arsenous acid (or arsenious acid) is the inorganic compound with the formula H3AsO3. It is known to occur in aqueous solutions, but it has not been isolated as a pure material, although this fact does not detract from the significance of As(OH)3. Properties As(OH)3 is a pyramidal molecule consisting of three hydroxyl groups bonded to arsenic. The 1H NMR spectrum of arsenous acid solutions consists of a single signal consistent with the molecule's high symmetry. In contrast, the nominally related phosphorous acid H3PO3 adopts the structure HPO(OH)2. The structural analogue of arsenous acid (P(OH)3) is a very minor equilibrium component of such solutions. The differing behaviors of the As and P compounds reflect a trend whereby high oxidation states are more stable for lighter members of main group elements than their heavier congeners. One tautomer of arsenous acid is HAsO(OH)2, which is called arsonic acid. It has not been isolated or well-characterized. Synthesis The preparat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Methyl Iodide
Iodomethane, also called methyl iodide, and commonly abbreviated "MeI", is the chemical compound with the formula CH3I. It is a dense, colorless, volatile liquid. In terms of chemical structure, it is related to methane by replacement of one hydrogen atom by an atom of iodine. It is naturally emitted by rice plantations in small amounts. It is also produced in vast quantities estimated to be greater than 214,000 tons annually by algae and kelp in the world's temperate oceans, and in lesser amounts on land by terrestrial fungi and bacteria. It is used in organic synthesis as a source of methyl groups. Preparation and handling Iodomethane is formed via the exothermic reaction that occurs when iodine is added to a mixture of methanol with red phosphorus. The iodinating reagent is phosphorus triiodide that is formed ''in situ:'' :3 CH3OH + PI3 → 3 CH3I + H2PO3H Alternatively, it is prepared from the reaction of dimethyl sulfate with potassium iodide in the presence of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oxidation State
In chemistry, the oxidation state, or oxidation number, is the hypothetical charge of an atom if all of its bonds to different atoms were fully ionic. It describes the degree of oxidation (loss of electrons) of an atom in a chemical compound. Conceptually, the oxidation state may be positive, negative or zero. While fully ionic bonds are not found in nature, many bonds exhibit strong ionicity, making oxidation state a useful predictor of charge. The oxidation state of an atom does not represent the "real" formal charge on that atom, or any other actual atomic property. This is particularly true of high oxidation states, where the ionization energy required to produce a multiply positive ion is far greater than the energies available in chemical reactions. Additionally, the oxidation states of atoms in a given compound may vary depending on the choice of electronegativity scale used in their calculation. Thus, the oxidation state of an atom in a compound is purely a formalism. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Biomethylation
In the chemical sciences, methylation denotes the addition of a methyl group on a substrate, or the substitution of an atom (or group) by a methyl group. Methylation is a form of alkylation, with a methyl group replacing a hydrogen atom. These terms are commonly used in chemistry, biochemistry, soil science, and the biological sciences. In biological systems, methylation is catalyzed by enzymes; such methylation can be involved in modification of heavy metals, regulation of gene expression, regulation of protein function, and RNA processing. In vitro methylation of tissue samples is also one method for reducing certain histological staining artifacts. The reverse of methylation is demethylation. In biology In biological systems, methylation is accomplished by enzymes. Methylation can modify heavy metals, regulate gene expression, RNA processing and protein function. It has been recognized as a key process underlying epigenetics. Methanogenesis Methanogenesis, the proces ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
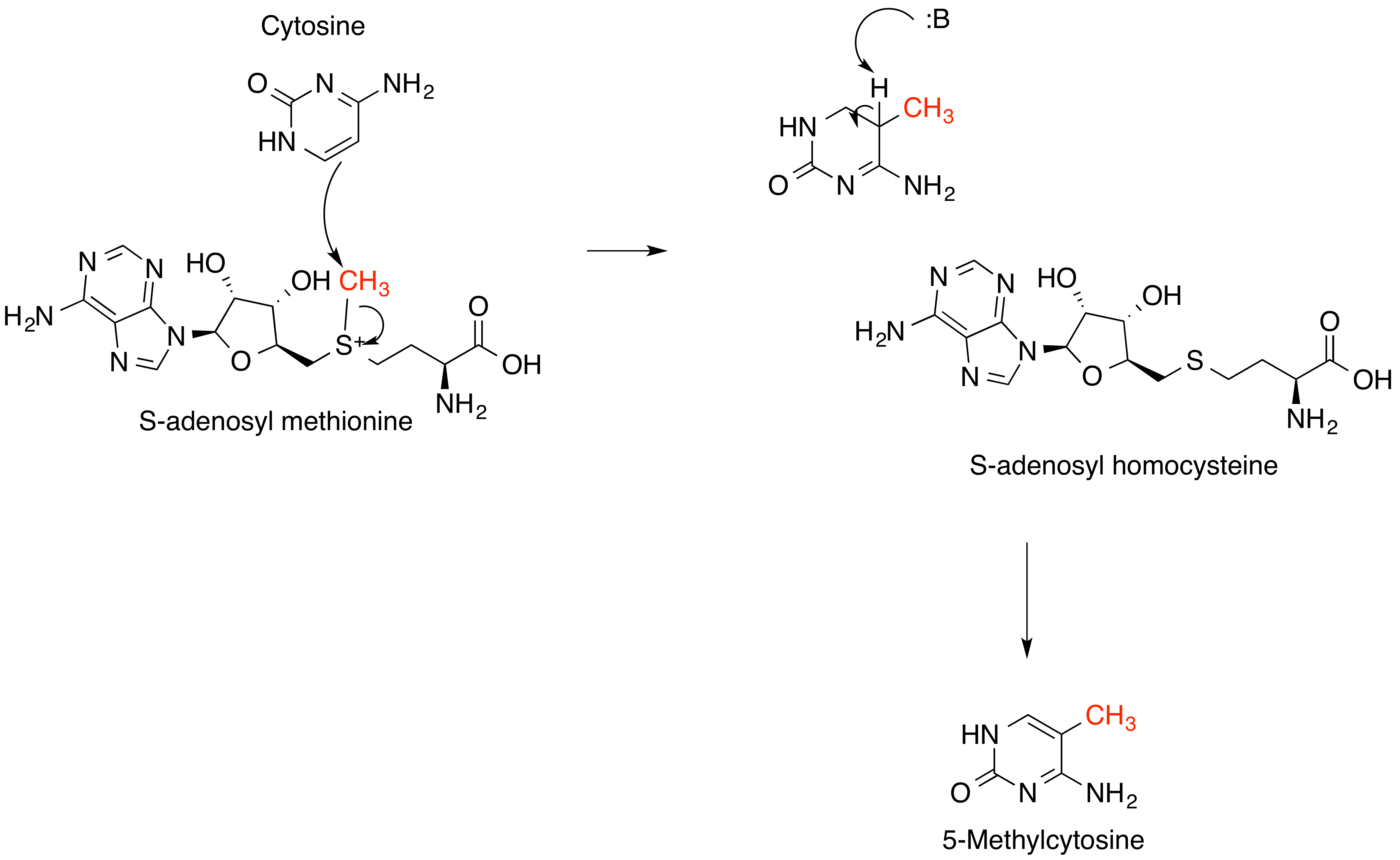
S-Adenosylmethionine
''S''-Adenosyl methionine (SAM), also known under the commercial names of SAMe, SAM-e, or AdoMet, is a common cosubstrate involved in methyl group transfers, transsulfuration, and aminopropylation. Although these anabolic reactions occur throughout the body, most SAM is produced and consumed in the liver. More than 40 methyl transfers from SAM are known, to various substrates such as nucleic acids, proteins, lipids and secondary metabolites. It is made from adenosine triphosphate (ATP) and methionine by methionine adenosyltransferase. SAM was first discovered by Giulio Cantoni in 1952. In bacteria, SAM is bound by the SAM riboswitch, which regulates genes involved in methionine or cysteine biosynthesis. In eukaryotic cells, SAM serves as a regulator of a variety of processes including DNA, tRNA, and rRNA methylation; immune response; amino acid metabolism; transsulfuration; and more. In plants, SAM is crucial to the biosynthesis of ethylene, an important plant hormone and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cacodylate
Cacodylic acid is an organoarsenic compound with the formula (CH3)2 AsO2H. With the formula R2As(O)OH, it is the simplest of the arsinic acids. It is a colorless solid that is soluble in water. Neutralization of cacodylic acid with base gives cacodylate salts, e.g. sodium cacodylate. They are potent herbicides. Cacodylic acid/sodium cacodylate is a buffering agent in the preparation and fixation of biological samples for electron microscopy. History In the 18th century it was found that combining and four equivalents of potassium acetate () gives a product called "Cadet's fuming liquid" which contains cacodyl oxide, and cacodyl, . Early research into "cacodyls" was reported by Robert Bunsen at the University of Marburg. Bunsen said of the compounds, "The smell of this body produces instantaneous tingling of the hands and feet, and even giddiness and insensibility... It is remarkable that when one is exposed to the smell of these compounds the tongue becomes covered with a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |