|
Metallacyclopentane
In organometallic chemistry, metallacyclopentanes are compounds with the formula LnM(CH2)4 (Ln = ligands, and M = metal). They are a type of metallacycle. Metallacyclopentanes are intermediates in some metal-catalysed reactions in homogeneous catalysis. Synthesis Traditionally, metallacyclopentanes are prepared by dialkylation of metal dihalides with 1,4‐bis(bromomagnesio)butane or the related dilithio reagent. The complex Ni(bipyridine)C4H8 is prepared by oxidative addition of 1,4-dibromobutane to Ni(0) precursors. Metallacyclopentanes also arise via the dimerization of ethylene within the coordination sphere of a low-valence metal center. This reaction is relevant to the catalytic production of butenes and related alkenes. Structure Unsubstituted metallacyclopentanes adopt conformations related to cyclopentane itself: open-envelope conformation and a twisted open-envelope structure. Occurrence Early examples of metallacyclopentanes come from studies of the Ni-catalyzed linea ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Metallacycle
In organometallic chemistry, a metallacycle is a derivative of a carbocyclic compound wherein a metal has replaced at least one carbon center; this is to some extent similar to heterocycles. Metallacycles appear frequently as reactive intermediates in catalysis, e.g. olefin metathesis and alkyne trimerization. In organic synthesis, directed ortho metalation is widely used for the functionalization of arene rings via C-H activation. One main effect that metallic atom substitution on a cyclic carbon compound is distorting the geometry due to the large size of typical metals. Nomenclature Typically, metallacycles are cyclic compounds with two metal carbon bonds. Many compounds containing metals in rings are known, for example chelate rings. Usually, such compounds are not classified as metallacycles, but the naming conventions are not rigidly followed. Within the area of coordination chemistry and supramolecular chemistry, examples include metallacrowns, metallacryptands, metalla ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
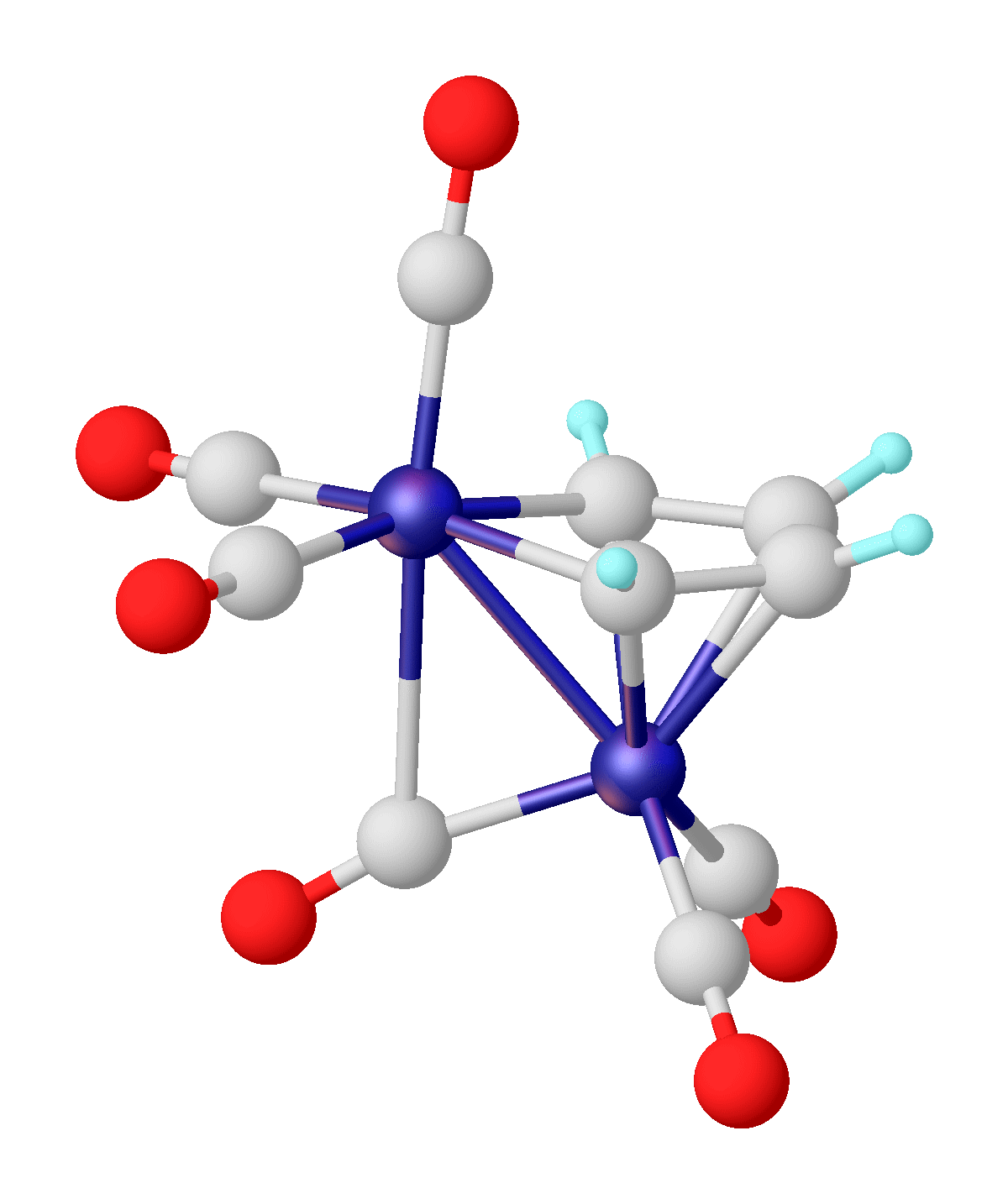
Metallacyclobutane
In organometallic chemistry, a metallacycle is a derivative of a carbocyclic compound wherein a metal has replaced at least one carbon center; this is to some extent similar to heterocycles. Metallacycles appear frequently as reactive intermediates in catalysis, e.g. olefin metathesis and alkyne trimerization. In organic synthesis, directed ortho metalation is widely used for the functionalization of arene rings via C-H activation. One main effect that metallic atom substitution on a cyclic carbon compound is distorting the geometry due to the large size of typical metals. Nomenclature Typically, metallacycles are cyclic compounds with two metal carbon bonds. Many compounds containing metals in rings are known, for example chelate rings. Usually, such compounds are not classified as metallacycles, but the naming conventions are not rigidly followed. Within the area of coordination chemistry and supramolecular chemistry, examples include metallacrowns, metallacryptands, metallah ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Metallole
Metalloles are derivatives of cyclopentadiene in which the carbon atom at position 5, the saturated carbon, is replaced by a heteroatom. In contrast to its parent compound, the numbering of the metallole starts at the heteroatom. Some of these compounds are described as organometallic compounds, but in the list below quite a number of metalloids are present too. Many metalloles are fluorescent. Polymeric derivatives of pyrrole and thiophene are of interest in molecular electronics. Metalloles, which can also be viewed as structural analogs of pyrrole, include: * Arsole, a moderately-aromatic arsenic analog * Bismole, a bismuth analog * Borole, a boron analog * Furan (oxole), an oxygen analog * Gallole, a gallium analog * Germole, a germanium analog * Phosphole, a phosphorus analog * Pyrrole (azole), a nitrogen analog * Selenophene, a selenium analog * Silole, a silicon analog * Stannole, a tin analog * Stibole, an antimony analog * Tellurophene, a tellurium analog *Plumbole, a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
1-octene
1-Octene is an organic compound with a formula CH2CHC6H13. The alkene is classified as a higher olefin and alpha-olefin, meaning that the double bond is located at the alpha (primary) position, endowing this compound with higher reactivity and thus useful chemical properties. 1-Octene is one of the important linear alpha olefins in industry. It is a colourless liquid. Synthesis In industry, 1-octene is commonly manufactured by two main routes: oligomerization of ethylene and by Fischer–Tropsch synthesis followed by purification. Another route to 1-octene that has been used commercially on a small scale is dehydration of alcohols. Prior to the 1970s, 1-octene was also manufactured by thermal cracking of waxes, whereas linear internal octenes were also manufactured by chlorination/dehydrochlorination of linear alkanes. There are five commercial processes that oligomerize ethylene to 1-octene. Four of these processes produce 1-octene as a part of a wide distribution of alpha-olefi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Metal Alkylidene
A metal (from Greek μέταλλον ''métallon'', "mine, quarry, metal") is a material that, when freshly prepared, polished, or fractured, shows a lustrous appearance, and conducts electricity and heat relatively well. Metals are typically ductile (can be drawn into wires) and malleable (they can be hammered into thin sheets). These properties are the result of the ''metallic bond'' between the atoms or molecules of the metal. A metal may be a chemical element such as iron; an alloy such as stainless steel; or a molecular compound such as polymeric sulfur nitride. In physics, a metal is generally regarded as any substance capable of conducting electricity at a temperature of absolute zero. Many elements and compounds that are not normally classified as metals become metallic under high pressures. For example, the nonmetal iodine gradually becomes a metal at a pressure of between 40 and 170 thousand times atmospheric pressure. Equally, some materials regarded as metals ca ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alkene Metathesis
Olefin metathesis is an organic reaction that entails the redistribution of fragments of alkenes (olefins) by the scission and regeneration of carbon-carbon double bonds. Because of the relative simplicity of olefin metathesis, it often creates fewer undesired by-products and hazardous wastes than alternative organic reactions. For their elucidation of the reaction mechanism and their discovery of a variety of highly active catalysts, Yves Chauvin, Robert H. Grubbs, and Richard R. Schrock were collectively awarded the 2005 Nobel Prize in Chemistry. Catalysts The reaction requires metal catalysts. Most commercially important processes employ heterogeneous catalysts. The heterogeneous catalysts are often prepared by in-situ activation of a metal halides (MClx) using organoaluminium or organotin compounds, e.g. combining MClx–EtAlCl2. A typical catalyst support is alumina. Commercial catalysts are often based on molybdenum and ruthenium. Well-defined organometallic compo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
The Chromium-catalyzed Trimerization
''The'' () is a grammatical article in English, denoting persons or things that are already or about to be mentioned, under discussion, implied or otherwise presumed familiar to listeners, readers, or speakers. It is the definite article in English. ''The'' is the most frequently used word in the English language; studies and analyses of texts have found it to account for seven percent of all printed English-language words. It is derived from gendered articles in Old English which combined in Middle English and now has a single form used with nouns of any gender. The word can be used with both singular and plural nouns, and with a noun that starts with any letter. This is different from many other languages, which have different forms of the definite article for different genders or numbers. Pronunciation In most dialects, "the" is pronounced as (with the voiced dental fricative followed by a schwa) when followed by a consonant sound, and as (homophone of the archaic pron ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |

.png)


.png)