|
Metal Dithiolene Complex
Dithiolene metal complexes are complexes containing 1,2-dithiolene ligands. 1,2-Dithiolene ligands, a particular case of 1,2-dichalcogenolene species, are unsaturated bidentate ligand wherein the two donor atoms are sulfur. 1,2-Dithiolene metal complexes are often referred to as "metal dithiolenes", "metallodithiolenes" or "dithiolene complexes". Most molybdenum- and tungsten-containing proteins have dithiolene-like moieties at their active sites, which feature the so-called molybdopterin cofactor bound to the Mo or W. Dithiolene metal complexes have been studied since the 1960s when they were first popularized by Gerhard N. Schrauzer and Volker P. Mayweg, who prepared nickel bis(stilbene-1,2-dithiolate) (Ni(S2C2Ph2)2) by the reaction of nickel sulfide and diphenylacetylene. The structural, spectroscopic, and electrochemical properties of many related complexes have been described. Structure and bonding Dithiolene metal complexes can be found in coordination compounds where ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mo(dith)3
Mo or MO may refer to: Arts and entertainment Fictional characters * Mo, a girl in the ''Horrible Histories (2001 TV series), Horrible Histories'' TV series * Mo, also known as Mortimer, in the novel ''Inkheart'' by Cornelia Funke * Mo, in the webcomic ''Jesus and Mo'' * Mo, the main character in the ''Mo's Mischief'' children's book series * Mo, an ophthalmosaurus from ''The Land Before Time (franchise), The Land Before Time'' franchise * MO (Maintenance Operator), a robot in the Filmation series ''Young Sentinels'' * Mo, a main character in ''Zoey's Extraordinary Playlist'' * M-O (Microbe Obliterator), a robot in film ''WALL-E'' * Mo the clown, a character played by Roy Rene, 20th-century Australian stage comedian * Mo Effanga, in the BBC medical drama series ''Holby City'' * Mo Harris, in the BBC soap opera ''EastEnders'' * Little Mo Mitchell, in the BBC soap opera ''EastEnders'' Films * "Mo" (魔 demon), original title of ''The Boxer's Omen'', a 1983 Hong Kong film * Mo (2 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Resonance Structures Of Dithiolene Complex
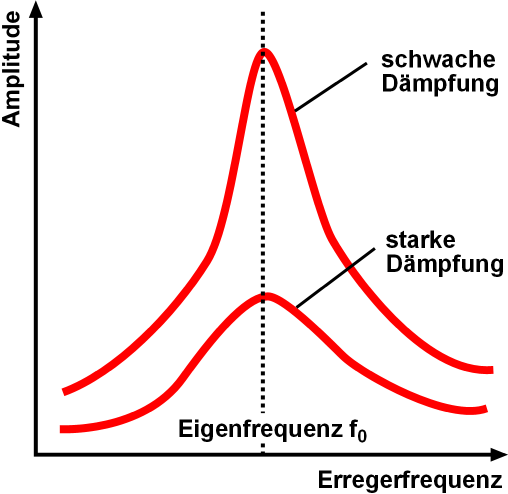
Resonance describes the phenomenon of increased amplitude that occurs when the frequency of an applied periodic force (or a Fourier component of it) is equal or close to a natural frequency of the system on which it acts. When an oscillating force is applied at a resonant frequency of a dynamic system, the system will oscillate at a higher amplitude than when the same force is applied at other, non-resonant frequencies. Frequencies at which the response amplitude is a relative maximum are also known as resonant frequencies or resonance frequencies of the system. Small periodic forces that are near a resonant frequency of the system have the ability to produce large amplitude oscillations in the system due to the storage of vibrational energy. Resonance phenomena occur with all types of vibrations or waves: there is mechanical resonance, orbital resonance, acoustic resonance, electromagnetic resonance, nuclear magnetic resonance (NMR), electron spin resonance (ESR) and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dithiete
Dithiete is an unsaturated heterocyclic compound that contains two adjacent sulfur atoms and two sp2-hybridized carbon centers. Derivatives are known collectively as dithietes or 1,2-dithietes. With 6 π electrons, 1,2-dithietes are examples of aromatic organosulfur compounds. A few 1,2-dithietes have been isolated. Unsubstituted 1,2-dithiete has been generated in thermolytic reactions and was characterized by microwave spectroscopy, ultraviolet photoelectron spectroscopy and infrared spectroscopy in a low temperature matrix. The open ring isomer, dithioglyoxal, HC(S)C(S)H, is less stable than the 1,2-dithiete. The dithione can be prepared (as ''trans''-dithioglyoxal) by low temperature photolysis of 1,3-dithiol-2-one. Quantum chemical calculations reproduce the observed greater stability of 1,2-dithiete only if large basis-sets with polarization functions are used. : See also * Dithietane - the corresponding saturated ring * Thiete Thiete is a heterocyclic compound contai ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
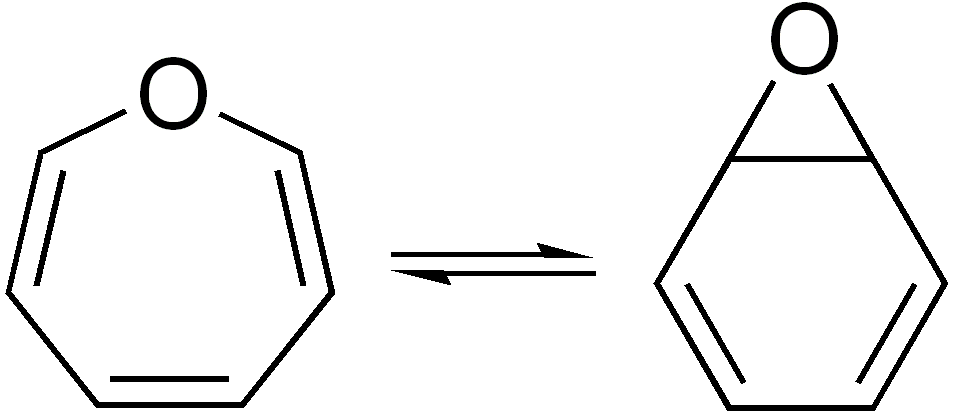
Valence Isomer
In organic chemistry, two molecules are valence isomers when they are constitutional isomers that can interconvert through pericyclic reactions. Benzene There are many valence isomers one can draw for the C6H6 formula benzene. Some were originally proposed for benzene itself before the actual structure of benzene was known. Others were later synthesized in lab. Some have been observed to isomerize to benzene, whereas others tend to undergo other reactions instead, or isomerize by ways other than pericyclic reactions. Image:Benzene-2D-flat.png, Benzene Image:Historic Benzene Formulae Dewar(1867) V.1.svg, Dewar benzene Image:Prisman2.svg, Prismane Image:Benzvalene.png, Benzvalene Image:Bicycloprop-2-enyl.svg, Bicyclopropenyl Cyclooctatetraene The valence isomers are not restricted to isomers of benzene. Valence isomers are also seen in the series (CH)8. Due to the larger number of units, the number of possible valence isomers is also greater and at least 21: Image:Cyclooctatet ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phosphorus Pentasulfide
Phosphorus pentasulfide is the inorganic compound with the formula (monomer) or (dimer). This yellow solid is the one of two phosphorus sulfides of commercial value. Samples often appear greenish-gray due to impurities. It is soluble in carbon disulfide but reacts with many other solvents such as alcohols, DMSO, and DMF. Structure and synthesis Its tetrahedral molecular structure is similar to that of adamantane and almost identical to the structure of phosphorus pentoxide. Phosphorus pentasulfide is obtained by the reaction of liquid white phosphorus () with sulfur above 300 °C. The first synthesis of by Berzelius in 1843 was by this method. Alternatively, can be formed by reacting elemental sulfur or pyrite, , with ferrophosphorus, a crude form of (a byproduct of white phosphorus () production from phosphate rock): : : Applications Approximately 150,000 tons of are produced annually. The compound is mainly converted to other derivatives for use as lubrication ad ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acyloin
Acyloins or α-hydroxy ketones are a class of organic compounds which all possess a hydroxy group adjacent to a ketone group. The name acyloin is derived from the fact that they are formally derived from reductive coupling of carboxylic acyl groups. Synthesis Classic organic reactions exist for the synthesis of acyloins. * The acyloin condensation is a reductive coupling of esters * The benzoin condensation is condensation reaction between aldehydes catalyzed by a nucleophile * Oxidation of carbonyls is possible with molecular oxygen but not selective * Better alternative is oxidation of corresponding silyl enol ethers with ''m''CPBA in the Rubottom oxidation * MoOPH oxidation of carbonyls is a system with molybdenum peroxide, pyridine and hexamethylphosphoramide. Enolate oxidation by sulfonyloxaziridines Enolates can be oxidized by sulfonyloxaziridines. The enolate reacts by nucleophilic displacement at the electron deficient oxygen of the oxaziridine ring. : This rea ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sodium Maleonitriledithiolate
Sodium maleonitriledithiolate is the chemical compound described by the formula Na2S2C2(CN)2. The name refers to the cis compound, structurally related to maleonitrile ((CHCN)2). Maleonitriledithiolate is often abbreviated mnt. It is a "dithiolene", i.e. a chelating alkene-1,2-dithiolate. It is a prototypical non-innocent ligand in coordination chemistry. Several complexes are known, such as i(mnt)2sup>2−. The salt is synthesized by treating carbon disulfide with sodium cyanide to give the cyanodithioformate salt, which eliminates elemental sulfur in aqueous solution: :2 NaCN + 2 CS2 → Na2S2C2(CN)2 + 1/4 S8 The compound was first described by Bähr and Schleitzer 1958. {{cite journal , author = G. Bähr and G. Schleitzer , title = Beiträge zur Chemie des Schwefelkohlenstoffs und Selenkohlenstoffs, II. Die Kondensierende Spontan-Entschwefelung von Salzen und Estern der Cyan-Dithioameisensäure. Freie Cyan-Dithioameisensäure , journal = Chemische Berichte ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sodium 1,3-dithiole-2-thione-4,5-dithiolate
Sodium 1,3-dithiole-2-thione-4,5-dithiolate is the organosulfur compound with the formula Na2C3S5, abbreviated Na2dmit. It is the sodium salt of the conjugate base of the 1,3-dithiole-2-thione-4,5-dithiol. The salt is a precursor to dithiolene complexes and tetrathiafulvalenes. Reduction of carbon disulfide with sodium affords sodium 1,3-dithiole-2-thione-4,5-dithiolate together with sodium trithiocarbonate: : 4 Na + 4 CS2 → Na2C3S5 + Na2CS3 Before the characterization of dmit2-, reduction of CS2 was thought to give tetrathiooxalate (Na2C2S4). The dianion C3S52- is purified as the tetraethylammonium salt of the zincate complex n(C3S5)2sup>2-. This salt converts to the bis(thioester) upon treatment with benzoyl chloride: : (C2H5)4sub>2 n(C3S5)2 + 4 C6H5COCl → 2 C3S3(SC(O)C6H5)2 + (C2H5)4sub>2 nCl4Cleavage of the thioester with sodium methoxide Sodium methoxide is the simplest sodium alkoxide. With the formula , it is a white solid, which is formed ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thiol
In organic chemistry, a thiol (; ), or thiol derivative, is any organosulfur compound of the form , where R represents an alkyl or other organic substituent. The functional group itself is referred to as either a thiol group or a sulfhydryl group, or a sulfanyl group. Thiols are the sulfur analogue of alcohols (that is, sulfur takes the place of oxygen in the hydroxyl () group of an alcohol), and the word is a blend of "''thio-''" with "alcohol". Many thiols have strong odors resembling that of garlic or rotten eggs. Thiols are used as odorants to assist in the detection of natural gas (which in pure form is odorless), and the "smell of natural gas" is due to the smell of the thiol used as the odorant. Thiols are sometimes referred to as mercaptans () or mercapto compounds, a term introduced in 1832 by William Christopher Zeise and is derived from the Latin ('capturing mercury')''Oxford American Dictionaries'' (Mac OS X Leopard). because the thiolate group () bonds very strong ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alkene
In organic chemistry, an alkene is a hydrocarbon containing a carbon–carbon double bond. Alkene is often used as synonym of olefin, that is, any hydrocarbon containing one or more double bonds.H. Stephen Stoker (2015): General, Organic, and Biological Chemistry'. 1232 pages. Two general types of monoalkenes are distinguished: terminal and internal. Also called α-olefins, terminal alkenes are more useful. However, the International Union of Pure and Applied Chemistry (IUPAC) recommends using the name "alkene" only for acyclic hydrocarbons with just one double bond; alkadiene, alkatriene, etc., or polyene for acyclic hydrocarbons with two or more double bonds; cycloalkene, cycloalkadiene, etc. for cyclic ones; and "olefin" for the general class – cyclic or acyclic, with one or more double bonds. Acyclic alkenes, with only one double bond and no other functional groups (also known as mono-enes) form a homologous series of hydrocarbons with the general formula with '' ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nonlinear Optics
Nonlinear optics (NLO) is the branch of optics that describes the behaviour of light in ''nonlinear media'', that is, media in which the polarization density P responds non-linearly to the electric field E of the light. The non-linearity is typically observed only at very high light intensities (when the electric field of the light is >108 V/m and thus comparable to the atomic electric field of ~1011 V/m) such as those provided by lasers. Above the Schwinger limit, the vacuum itself is expected to become nonlinear. In nonlinear optics, the superposition principle no longer holds. History The first nonlinear optical effect to be predicted was two-photon absorption, by Maria Goeppert Mayer for her PhD in 1931, but it remained an unexplored theoretical curiosity until 1961 and the almost simultaneous observation of two-photon absorption at Bell Labs and the discovery of second-harmonic generation by Peter Franken ''et al.'' at University of Michigan, both shortly after the constru ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
2Ni(mnt)2.jpg)