|
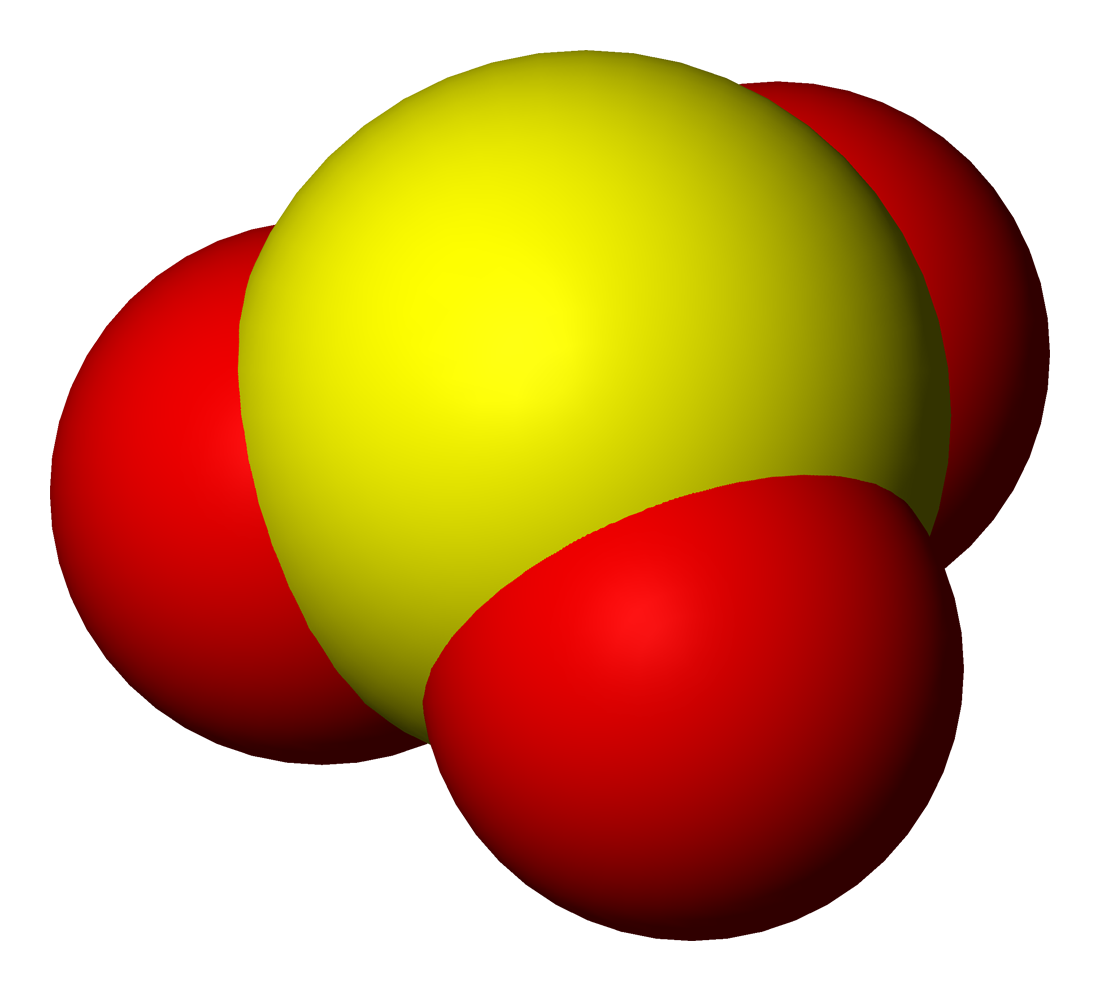
Metabisulfite
A disulfite, commonly known as metabisulfite or pyrosulfite, is a chemical compound containing the ion . It is a colorless dianion that is primarily marketed in the form of sodium metabisulfite or potassium metabisulfite. When dissolved in water, these salts release the hydrogensulfite anion. These salts act equivalently to sodium hydrogensulfite or potassium hydrogensulfite. Structure In contrast to disulfate (), disulfite ion () has an unsymmetrical structure with an S-S bond. The oxidation state of the sulfur atom bonded to 3 oxygen atoms is +5 while oxidation number of other sulfur atom is +3. The anion consists of an SO2 group linked to an SO3 group, with the negative charge more localized on the SO3 end. The S–S bond length is 2.22 Å, and the "thionate" and "thionite" S–O distances are 1.46 and 1.50 Å respectively. Production Salts of disulfite ion are produced by dehydration of salts of hydrogensulfite ion (). When solutions of sodium hydrogensu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Potassium Metabisulphite
Potassium metabisulfite, K2S2O5, also known as potassium pyrosulfite, is a white crystalline powder with a pungent odour. It is mainly used as an antioxidant or Sterilization (microbiology)#Chemical sterilization, chemical sterilant. As a disulfite, it is chemically very similar to sodium metabisulfite, with which it is sometimes used interchangeably. Potassium metabisulfite has a monoclinic crystal structure. Preparation and reactions Potassium metabisulfite can be prepared by treating a solution of potassium hydroxide with sulfur dioxide. :2 SO2 + 2 KOH → K2S2O5 + H2O It decomposes at 190 °C, yielding potassium sulfite and sulfur dioxide: :K2S2O5 → K2SO3 + SO2 Uses It is used as a food additive, also known as E224. It is restricted in use and may cause allergic reactions in some sensitive persons. Wine Potassium metabisulfite is a common wine or must additive, in which it forms sulfur dioxide (SO2). Sulfur dioxide is a disinfectant. It also acts as a poten ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Potassium Metabisulfite
Potassium metabisulfite, K2S2O5, also known as potassium pyrosulfite, is a white crystalline powder with a pungent odour. It is mainly used as an antioxidant or chemical sterilant. As a disulfite, it is chemically very similar to sodium metabisulfite, with which it is sometimes used interchangeably. Potassium metabisulfite has a monoclinic crystal structure. Preparation and reactions Potassium metabisulfite can be prepared by treating a solution of potassium hydroxide with sulfur dioxide. :2 SO2 + 2 KOH → K2S2O5 + H2O It decomposes at 190 °C, yielding potassium sulfite and sulfur dioxide: :K2S2O5 → K2SO3 + SO2 Uses It is used as a food additive, also known as E224. It is restricted in use and may cause allergic reactions in some sensitive persons. Wine Potassium metabisulfite is a common wine or must additive, in which it forms sulfur dioxide (SO2). Sulfur dioxide is a disinfectant. It also acts as a potent antioxidant, protecting both the color and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sodium Metabisulfite
Sodium metabisulfite or sodium pyrosulfite (IUPAC spelling; Br. E. sodium metabisulphite or sodium pyrosulphite) is an inorganic compound of chemical formula Na2S2O5. The substance is sometimes referred to as disodium metabisulfite. It is used as a disinfectant, antioxidant, and preservative agent. Preparation Sodium metabisulfite can be prepared by treating a solution of sodium hydroxide with sulfur dioxide. When conducted in warm water, Na2SO3 initially precipitates as a yellow solid. With more SO2, the solid dissolves to give the disulfite, which crystallises upon cooling. :SO2 + 2 NaOH → Na2SO3 + H2O :SO2 + Na2SO3 → Na2S2O5 which yields a residue of colourless solid Na2S2O5. Chemical structure The anion metabisulfite consists of an SO2 group linked to an SO3 group, with the negative charge more localised on the SO3 end. The S–S bond length is 2.22 Å, and the "thionate" and "thionite" S–O distances are 1.46 and 1.50 Å, respectively. Reactivity Upon ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sodium Metabisulphite
Sodium metabisulfite or sodium pyrosulfite (IUPAC spelling; Br. E. sodium metabisulphite or sodium pyrosulphite) is an inorganic compound of chemical formula Na2S2O5. The substance is sometimes referred to as disodium metabisulfite. It is used as a disinfectant, antioxidant, and preservative agent. Preparation Sodium metabisulfite can be prepared by treating a solution of sodium hydroxide with sulfur dioxide. When conducted in warm water, Na2SO3 initially precipitates as a yellow solid. With more SO2, the solid dissolves to give the disulfite, which crystallises upon cooling. :SO2 + 2 NaOH → Na2SO3 + H2O :SO2 + Na2SO3 → Na2S2O5 which yields a residue of colourless solid Na2S2O5. Chemical structure The anion metabisulfite consists of an SO2 group linked to an SO3 group, with the negative charge more localised on the SO3 end. The S–S bond length is 2.22 Å, and the "thionate" and "thionite" S–O distances are 1.46 and 1.50 Å, respectively. Reactivity U ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sulfite Food And Beverage Additives
The topic of sulfite food and beverage additives covers the application of sulfites in food chemistry. "Sulfite" is jargon that encompasses a variety of materials that are commonly used as preservatives or food additive in the production of diverse foods and beverages. Although sulfite salts are relatively nontoxic, their use has led to controversy, resulting in extensive regulations. Sulfites are a source of sulfur dioxide (SO2), a bactericide. Chemical principles Inventory *sodium bisulfite, NaHSO3: ill-defined, widely used source of bisulfite, which predominates below pH 7 *sodium metabisulfite, Na2S2O5: well-defined, widely used source of bisulfite, which predominates below pH 7 *potassium bisulfite, KHSO3: ill-defined, widely used source of bisulfite, which predominates below pH 7 *potassium metabisulfite, K2S2O5,: well-defined, widely used source of bisulfite, which predominates below pH 7 *sodium sulfite, Na2SO3: well-defined, widely used source of sulfite, which predomina ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sulfite
Sulfites or sulphites are compounds that contain the sulfite ion (or the sulfate(IV) ion, from its correct systematic name), . The sulfite ion is the conjugate base of bisulfite. Although its acid ( sulfurous acid) is elusive, its salts are widely used. Sulfites are substances that naturally occur in some foods and the human body. They are also used as regulated food additives. When in food or drink, sulfites are often lumped together with sulfur dioxide.SeREGULATION (EU) No 1169/2011 OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL/ref> Structure The structure of the sulfite anion can be described with three equivalent resonance structures. In each resonance structure, the sulfur atom is double-bonded to one oxygen atom with a formal charge of zero (neutral), and sulfur is singly bonded to the other two oxygen atoms, which each carry a formal charge of −1, together accounting for the −2 charge on the anion. There is also a non-bonded lone pair on the sulfur, so the st ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sodium Hydrogen Sulfite
Sodium bisulfite (or sodium bisulphite, sodium hydrogen sulfite) is a chemical mixture with the approximate chemical formula NaHSO3. Sodium bisulfite in fact is not a real compound, but a mixture of salts that dissolve in water to give solutions composed of sodium and bisulfite ions. It appears in form of white or yellowish-white crystals with an odor of sulfur dioxide. For properties of sodium bisulfite, refer to the table located to the right. Regardless of its ill-defined nature, sodium bisulfite is used in many different industries such a food additive with E number E222 in the food industry, a reducing agent in the cosmetic industry, and a decomposer of residual hypochlorite used in the bleaching industry. Synthesis Sodium bisulfite solutions can be prepared by treating a solution of suitable base, such as sodium hydroxide or sodium bicarbonate with sulfur dioxide. :SO2 + NaOH → NaHSO3 :SO2 + NaHCO3 → NaHSO3 + CO2 Attempts to crystallize the product yield Sod ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrogensulfite
The bisulfite ion (IUPAC-recommended nomenclature: hydrogensulfite) is the ion . Salts containing the ion are also known as "sulfite lyes". Sodium bisulfite is used interchangeably with sodium metabisulfite (Na2S2O5). Sodium metabisulfite dissolves in water to give a solution of Na+. :Na2S2O5 + H2O → 2Na SO3 Structure The bisulfite anion exists in solution as a mixture of two tautomers. One tautomer has the proton attached to one of the three oxygen centers. In the second tautomer the proton resides on sulfur. The S-protonated tautomer has ''C''3v symmetry. The O-protonated tautomer has only Cs symmetry. Reactions Tautomerization There exist two tautomers of bisulfite. They interconvert readily but can be characterized individually by various spectroscopic methods. They have been observed by 17O NMR spectroscopy: :HSO3− SO2(OH)− K = 4.2 Acid-base reactions Solutions of bisulfite are typically prepared by treatment of sulfur dioxide with aqueous base: :S ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sodium Bisulfite
Sodium bisulfite (or sodium bisulphite, sodium hydrogen sulfite) is a chemical mixture with the approximate chemical formula NaHSO3. Sodium bisulfite in fact is not a real compound, but a mixture of salts that dissolve in water to give solutions composed of sodium and bisulfite ions. It appears in form of white or yellowish-white crystals with an odor of sulfur dioxide. For properties of sodium bisulfite, refer to the table located to the right. Regardless of its ill-defined nature, sodium bisulfite is used in many different industries such a food additive with E number E222 in the food industry, a reducing agent in the cosmetic industry, and a decomposer of residual hypochlorite used in the bleaching industry. Synthesis Sodium bisulfite solutions can be prepared by treating a solution of suitable base, such as sodium hydroxide or sodium bicarbonate with sulfur dioxide. :SO2 + NaOH → NaHSO3 :SO2 + NaHCO3 → NaHSO3 + CO2 Attempts to crystallize the product yield So ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
E Number
E numbers ("E" stands for "Europe") are codes for substances used as food additives, including those found naturally in many foods such as vitamin C, for use within the European Union (EU) and European Free Trade Association (EFTA). Commonly found on food labels, their safety assessment and approval are the responsibility of the European Food Safety Authority (EFSA). The fact that an additive has an E number implies that its use was at one time permitted in products for sale in the European Single Market; some of these additives are no longer allowed today. Having a single unified list for food additives was first agreed upon in 1962 with food colouring. In 1964, the directives for preservatives were added, in 1970 antioxidants were added, in 1974 emulsifiers, stabilisers, thickeners and gelling agents were added as well. Numbering schemes The numbering scheme follows that of the International Numbering System (INS) as determined by the ''Codex Alimentarius'' committee, t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Disulfurous Acid
Disulfurous acid or pyrosulfurous acid is an oxoacid of sulfur with the formula H2S2O5. The salts of disulfurous acid are called disulfites or metabisulfites. Disulfurous acid is, like sulfurous acid (H2SO3), a phantom acid, which does not exist in the free state. In contrast to disulfate (), disulfite has two directly connected sulfur atoms. The oxidation state In chemistry, the oxidation state, or oxidation number, is the hypothetical charge of an atom if all of its bonds to different atoms were fully ionic. It describes the degree of oxidation (loss of electrons) of an atom in a chemical compound. C ... of the sulfur atom bonded to three oxygen atoms is +5 while that of the other is +3. References Sulfur oxoacids Metabisulfites Hypothetical chemical compounds {{theoretical-chem-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Potassium Hydrogen Sulfite
Potassium bisulfite (or potassium hydrogen sulfite) is a chemical mixture with the approximate chemical formula KHSO3. Potassium bisulfite in fact is not a real compound, but a mixture of salts that dissolve in water to give solutions composed of potassium ions and bisulfite ions. It is a white solid with an odor of sulfur dioxide. Attempts to crystallize potassium bisulfite yield potassium metabisulfite, K2S2O5. Potassium bisulfite is used as a sterilising agent in the production of alcoholic beverages. This additive is classified as E number E228 under the current EU-approved food additive legislation. Production It is made by the reaction of sulfur dioxide and potassium carbonate. The sulfur dioxide is passed through a solution of the potassium carbonate until no more carbon dioxide is evolved. The solution is concentrated. See also * Calcium bisulfite * Sodium bisulfite Sodium bisulfite (or sodium bisulphite, sodium hydrogen sulfite) is a chemical mixture with t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |