|
Mesityl Oxide
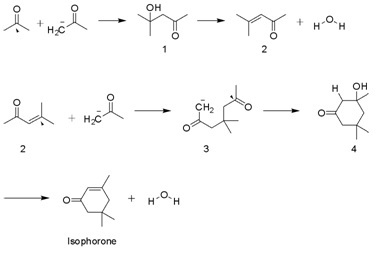
Mesityl oxide is a Carbonyl#.CE.B1.2C.CE.B2-Unsaturated_carbonyl_compounds, α,β-unsaturated ketone with the formula CH3C(O)CH=C(CH3)2. This compound is a colorless, volatile liquid with a honey-like odor. Synthesis It is prepared by the aldol condensation of acetone to give diacetone alcohol, which readily dehydrates to give this compound. : Phorone and isophorone may be formed under the same conditions. Isophorone originates via a Michael addition: : Phorone is formed by continued aldol condensation: : Uses Mesityl oxide is used as a solvent and in the production of methyl isobutyl ketone by hydrogenation: : Further hydrogenation gives 4-methyl-2-pentanol (methyl isobutyl carbinol). Dimedone is another established use of mesityl oxide. References {{reflist External linksIPCS INCHEM Description Enones Ketone solvents ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Michael Addition
In organic chemistry, the Michael reaction or Michael addition is a reaction between a Michael donor (an enolate or other nucleophile) and a Michael acceptor (usually an α,β-unsaturated carbonyl) to produce a Michael adduct by creating a carbon-carbon bond at the acceptor's β-carbon. It belongs to the larger class of conjugate additions and is widely used for the mild formation of carbon-carbon bonds. The Michael addition is an important atom-economical method for diastereoselective and enantioselective C–C bond formation, and many asymmetric variants exist : In this general Michael addition scheme, either or both of R and R' on the nucleophile (the Michael donor) represent electron-withdrawing substituents such as acyl, cyano, nitro, or sulfone groups, which make the adjacent methylene hydrogen acidic enough to form a carbanion when reacted with the base, ''B:''. For the alkene (the Michael acceptor), the R" substituent is usually a carbonyl, which makes the compound ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dimedone
Dimedone is a cyclic diketone used in organic chemistry to determine whether a compound contains an aldehyde group. Cyclohexanediones in general can be used as catalysts in the formation of transition-metal complexes. Other uses include applications in colorimetry, crystallography, luminescence and spectrophotometric analysis. It can also be used for chemistry involving organic compounds of low electrical resistance. Synthesis Dimedone is prepared from mesityl oxide and diethyl malonate. Physical properties Dimedone usually comes in the form of white crystals. It is stable under ambient conditions and soluble in water, as well as ethanol and methanol. It has a melting point range of 147–150 °C (420–423 K). Chemical properties Tautomerism Dimedone is in equilibrium with its tautomer Tautomers () are structural isomers (constitutional isomers) of chemical compounds that readily interconvert. The chemical reaction interconverting the two is called tautomeriz ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
4-methyl-2-pentanol
4-Methyl-2-pentanol (IUPAC name: 4-methylpentan-2-ol) or methyl isobutyl carbinol (MIBC) is an organic chemical compound used primarily as a frother in mineral flotation and in the production of lubricant oil additives such as Zinc dithiophosphate. It is also used as a solvent, in organic synthesis, and in the manufacture of brake fluid and as a precursor to some plasticizer A plasticizer ( UK: plasticiser) is a substance that is added to a material to make it softer and more flexible, to increase its plasticity, to decrease its viscosity, and/or to decrease friction during its handling in manufacture. Plasticiz ...s. It is an acetone derivative in liquid state, with limited solubility in water but generally miscible with most organic solvents. References Hexanols {{mining-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrogenation Of Mesityl Oxide To MIBK
Hydrogenation is a chemical reaction between molecular hydrogen (H2) and another compound or element, usually in the presence of a catalyst such as nickel, palladium or platinum. The process is commonly employed to reduce or saturate organic compounds. Hydrogenation typically constitutes the addition of pairs of hydrogen atoms to a molecule, often an alkene. Catalysts are required for the reaction to be usable; non-catalytic hydrogenation takes place only at very high temperatures. Hydrogenation reduces double and triple bonds in hydrocarbons. Process Hydrogenation has three components, the unsaturated substrate, the hydrogen (or hydrogen source) and, invariably, a catalyst. The reduction reaction is carried out at different temperatures and pressures depending upon the substrate and the activity of the catalyst. Related or competing reactions The same catalysts and conditions that are used for hydrogenation reactions can also lead to isomerization of the alkenes from cis ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrogenation
Hydrogenation is a chemical reaction between molecular hydrogen (H2) and another compound or element, usually in the presence of a Catalysis, catalyst such as nickel, palladium or platinum. The process is commonly employed to redox, reduce or Saturated and unsaturated compounds, saturate organic compounds. Hydrogenation typically constitutes the addition of pairs of hydrogen atoms to a molecule, often an alkene. Catalysts are required for the reaction to be usable; non-catalytic hydrogenation takes place only at very high temperatures. Hydrogenation reduces Double bond, double and Triple bond, triple bonds in hydrocarbons. Process Hydrogenation has three components, the Saturated and unsaturated compounds, unsaturated substrate, the hydrogen (or hydrogen source) and, invariably, a catalyst. The redox, reduction reaction is carried out at different temperatures and pressures depending upon the substrate and the activity of the catalyst. Related or competing reactions The same ca ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Methyl Isobutyl Ketone
Methyl isobutyl ketone (MIBK) is the common name for the organic compound 4-methylpentan-2-one, condensed chemical formula (CH3)2CHCH2C(O)CH3. This colourless liquid, a ketone, is used as a solvent for gums, resins, paints, varnishes, lacquers, and nitrocellulose. Production At laboratory scale, MIBK can be produced via a three-step process using acetone as the starting material. Self-condensation, a type of aldol reaction, produces diacetone alcohol, which readily dehydrates to give 4-methylpent-3-en-2-one (commonly, mesityl oxide). Mesityl oxide is then hydrogenated to give MIBK. : Industrially, these three steps are combined. Acetone is treated with a strongly acidic, palladium catalyst-doped cation exchange resin under medium pressure of hydrogen. Several million kilograms are produced annually.Stylianos Sifniades, Alan B. Levy, "Acetone" in ''Ullmann’s Encyclopedia of Industrial Chemistry'', Wiley-VCH, Weinheim, 2005. Uses MIBK is used as a solvent for nitrocellulose, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phoron Formation
Tempering is a cooking technique used in India, Bangladesh, Nepal, Pakistan and Sri Lanka, in which whole spices (and sometimes also other ingredients such as dried chillies, minced ginger root or sugar) are roasted briefly in oil or ghee to liberate essential oils from cells and thus enhance their flavours, before being poured, together with the oil, into a dish. Tempering is also practiced by dry-roasting whole spices in a pan before grinding the spices. Tempering is typically done at the beginning of cooking, before adding the other ingredients for a curry or similar dish, or it may be added to a dish at the end of cooking, just before serving (as with a dal, sambar or stew). Ingredients used Ingredients typically used in tempering include cumin seeds, black mustard seeds, fennel seeds, '' kalonji'', fresh green chilis, dried red chilis, fenugreek seeds, asafoetida, cassia, cloves, urad dal, curry leaves, chopped onion, garlic, or tejpat leaves. When using multiple ingred ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isophorone Mesityl Oxide
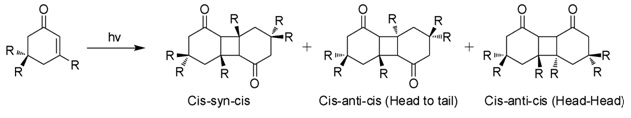
Isophorone is an α,β-unsaturated cyclic ketone. It is a colorless liquid with a characteristic peppermint-like odor, although commercial samples can appear yellowish. Used as a solvent and as a precursor to polymers, it is produced on a large scale industrially. Structure and reactivity Isophorone undergoes reactions characteristic of an α,β-unsaturated ketone. Hydrogenation gives the cyclohexanone derivative. Epoxidation with basic hydrogen peroxide affords the oxide. Isophorone is degraded by attack of hydroxyl radicals. Photodimerization When exposed to sunlight in aqueous solutions, isophorone undergoes 2+2 photocycloaddition to give three isomeric photodimers (Figure). These "diketomers" are cis-syn-cis, head to tail (HT), cys-anti-cys (HT), and head-head (HH). The formation of HH photodimers is favored over HT photodimers with increasing polarity of the medium. Natural Occurrence Isophorone occurs naturally in cranberries. Synthesis Isophorone is pro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isophorone
Isophorone is an Alpha-beta Unsaturated carbonyl compounds, α,β-unsaturated cyclic ketone. It is a colorless liquid with a characteristic peppermint-like odor, although commercial samples can appear yellowish. Used as a solvent and as a precursor to polymers, it is produced on a large scale industrially. Structure and reactivity Isophorone undergoes reactions characteristic of an α,β-unsaturated ketone. Hydrogenation gives the cyclohexanone derivative. Epoxidation with basic hydrogen peroxide affords the oxide. Isophorone is degraded by attack of hydroxyl radicals. Photodimerization When exposed to sunlight in aqueous solutions, isophorone undergoes 2+2 photocycloaddition to give three isomeric photodimers (Figure). These "diketomers" are cis-syn-cis, head to tail (HT), cys-anti-cys (HT), and head-head (HH). The formation of HH photodimers is favored over HT photodimers with increasing polarity of the medium. Natural Occurrence Isophorone occurs naturally in cran ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acetone
Acetone (2-propanone or dimethyl ketone), is an organic compound with the formula . It is the simplest and smallest ketone (). It is a colorless, highly volatile and flammable liquid with a characteristic pungent odour. Acetone is miscible with water and serves as an important organic solvent in its own right, in industry, home, and laboratory. About 6.7 million tonnes were produced worldwide in 2010, mainly for use as a solvent and production of methyl methacrylate (and from that PMMA) as well as bisphenol A.Acetone World Petrochemicals report, January 2010Stylianos Sifniades, Alan B. Levy, "Acetone" in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim, 2005. It is a common building block in |
Phorone
Phorone, or diisopropylidene acetone, is a yellow crystalline substance with a geranium odor, with formula or . Preparation It was first obtained in 1837 in impure form by the French chemist Auguste Laurent, who called it "camphoryle". In 1849, the French chemist Charles Frédéric Gerhardt and his student Jean Pierre Liès-Bodart prepared it in a pure state and named it "phorone". On both occasions it was produced by ketonization through the dry distillation of the calcium salt of camphoric acid. : It is now typically obtained by the acid-catalysed twofold aldol condensation of three molecules of acetone. Mesityl oxide is obtained as an intermediate and can be isolated. Crude phorone can be purified by repeated recrystallization from ethanol or ether, in which it is soluble. Reactions Phorone can condense with ammonia to form triacetone amine. See also *Isophorone Isophorone is an α,β-unsaturated cyclic ketone. It is a colorless liquid with a characteristic peppe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |