|
Merrifield Resin
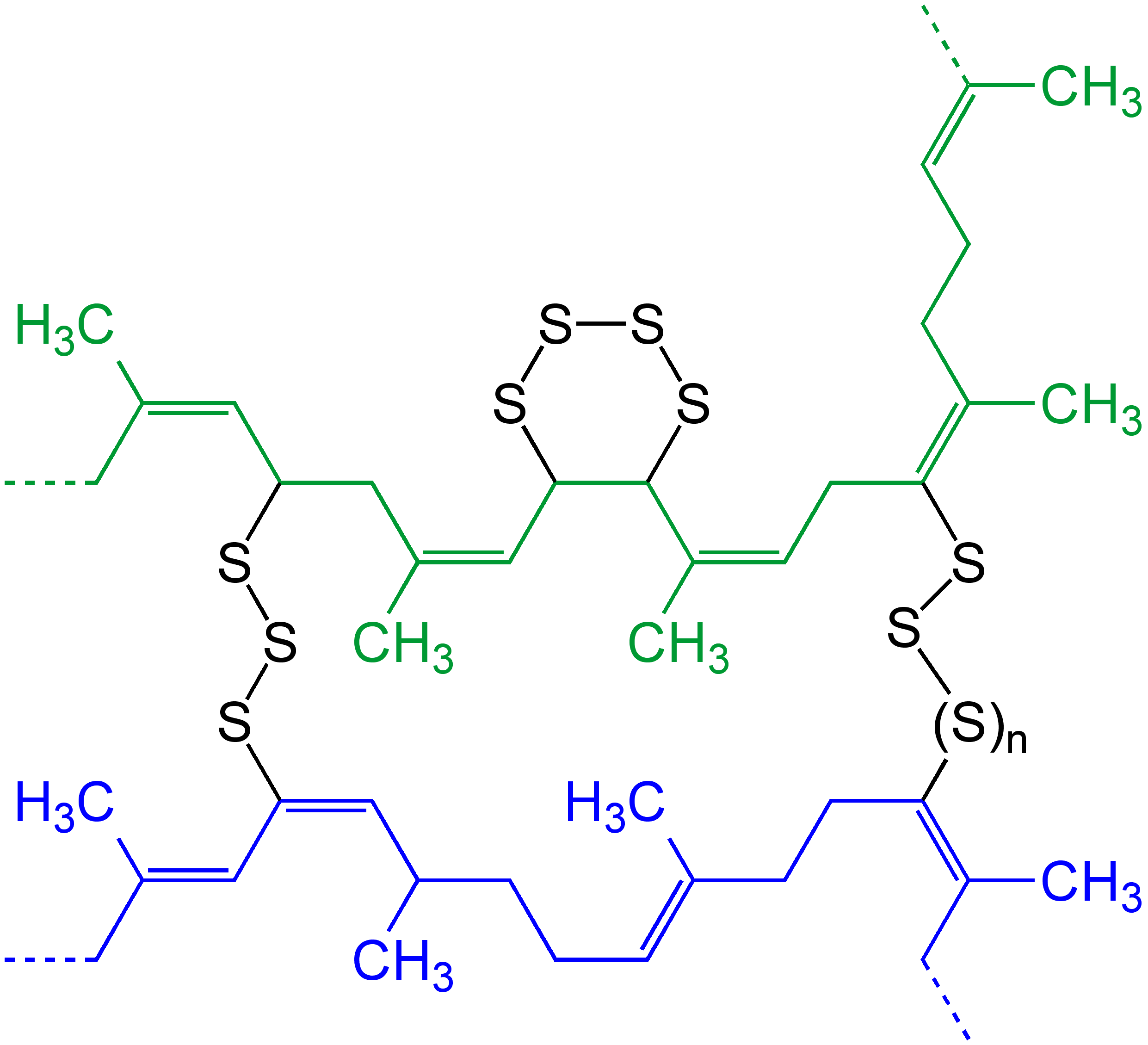
Merrifield Resin is a cross-linked polystyrene resin that carries a chloromethyl functional group. Merrifield resin is named after its inventor, Robert Bruce Merrifield (1984 winner of the Nobel Prize in Chemistry), and used in solid-phase synthesis. The material is typically available as white beads. These beads are allowed to swell in suitable solvents (ethyl acetate, DMF, DMSO), which then allows the reagents to substitute the chloride substituents.Vaino, Andrew R.; Janda, Kim D. "Solid-Phase Organic Synthesis: A Critical Understanding of the Resin" Journal of Combinatorial Chemistry 2000, volume 2, 579-596. Merrifield Resin can be prepared by chloromethylation of polystyrene or by the copolymerization of styrene Styrene () is an organic compound with the chemical formula C6H5CH=CH2. This derivative of benzene is a colorless oily liquid, although aged samples can appear yellowish. The compound evaporates easily and has a sweet smell, although high concen ... and 4-vinylbe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cross-link
In chemistry and biology a cross-link is a bond or a short sequence of bonds that links one polymer chain to another. These links may take the form of covalent bonds or ionic bonds and the polymers can be either synthetic polymers or natural polymers (such as proteins). In polymer chemistry "cross-linking" usually refers to the use of cross-links to promote a change in the polymers' physical properties. When "crosslinking" is used in the biological field, it refers to the use of a probe to link proteins together to check for protein–protein interactions, as well as other creative cross-linking methodologies. Although the term is used to refer to the "linking of polymer chains" for both sciences, the extent of crosslinking and specificities of the crosslinking agents vary greatly. As with all science, there are overlaps, and the following delineations are a starting point to understanding the subtleties. Polymer chemistry Crosslinking is the general term for the process of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polystyrene
Polystyrene (PS) is a synthetic polymer made from monomers of the aromatic hydrocarbon styrene. Polystyrene can be solid or foamed. General-purpose polystyrene is clear, hard, and brittle. It is an inexpensive resin per unit weight. It is a poor barrier to oxygen and water vapour and has a relatively low melting point. Polystyrene is one of the most widely used plastics, the scale of its production being several million tonnes per year. Polystyrene can be naturally transparent, but can be colored with colorants. Uses include protective packaging (such as packing peanuts and in the jewel cases used for storage of optical discs such as CDs and occasionally DVDs), containers, lids, bottles, trays, tumblers, disposable cutlery, in the making of models, and as an alternative material for phonograph records. As a thermoplastic polymer, polystyrene is in a solid (glassy) state at room temperature but flows if heated above about 100 °C, its glass transition temperature. I ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
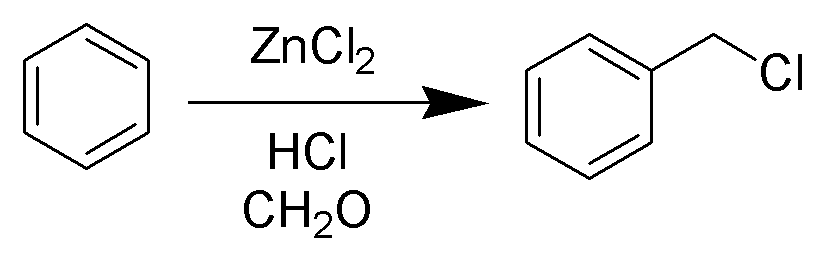
Chloromethyl
The Blanc chloromethylation (also called the Blanc reaction) is the chemical reaction of aromatic rings with formaldehyde and hydrogen chloride to form chloromethyl arenes. The reaction is catalyzed by Lewis acids such as zinc chloride. The reaction was discovered by Gustave Louis Blanc (1872-1927) in 1923 Mechanism and scope The reaction is carried out under acidic conditions and with a ZnCl2 catalyst. These conditions protonate the formaldehyde carbonyl making the carbon much more electrophilic. The aldehyde is then attacked by the aromatic pi-electrons, followed by rearomatization of the aromatic ring. The benzyl alcohol thus formed is quickly converted to the chloride under the reaction conditions. Other possibilities for the electrophile include (chloromethyl)oxonium cation (ClH2C–OH2+) or chlorocarbenium cation (ClCH2+), which may be formed in the presence of zinc chloride. These species may account for the fact that moderately and strongly deactivated substrates th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Robert Bruce Merrifield
Robert Bruce Merrifield (July 15, 1921 – May 14, 2006) was an American biochemist who won the Nobel Prize in Chemistry in 1984 for the invention of solid phase peptide synthesis. Early life He was born in Fort Worth, Texas, on 15 July 1921, the only son of George E. Merrifield and Lorene née Lucas. In 1923 the family moved to California where he attended nine grade schools and two high schools before graduating from Montebello High School in 1939. It was there that he developed an interest both in chemistry and in astronomy. After two years at Pasadena Junior College he transferred to the University of California at Los Angeles (UCLA). After graduation in chemistry he worked for a year at the Philip R. Park Research Foundation taking care of an animal colony and assisting with growth experiments on synthetic amino acid diets. One of these was the experiment by Geiger that first demonstrated that the essential amino acids must be present simultaneously for growth to occur. He ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Solid-phase Synthesis
In chemistry, solid-phase synthesis is a method in which molecules are covalently bound on a solid support material and synthesised step-by-step in a single reaction vessel utilising selective protecting group chemistry. Benefits compared with normal synthesis in a liquid state include: * High efficiency and throughput * Increased simplicity and speed The reaction can be driven to completion and high yields through the use of excess reagent. In this method, building blocks are protected at all reactive functional groups. The order of functional group reactions can be controlled by the order of deprotection. This method is used for the synthesis of peptides, deoxyribonucleic acid ( DNA), ribonucleic acid (RNA), and other molecules that need to be synthesised in a certain alignment. More recently, this method has also been used in combinatorial chemistry and other synthetic applications. The process was originally developed in the 1950s and 1960s by Robert Bruce Merrifield in orde ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
ACS Combinatorial Science
''ACS Combinatorial Science'' (usually abbreviated as ''ACS Comb. Sci.''), formerly ''Journal of Combinatorial Chemistry'' (1999-2010), was a peer-reviewed scientific journal, published since 1999 by the American Chemical Society. ''ACS Combinatorial Science'' publishes articles, reviews, perspectives, accounts and reports in the field of Combinatorial Chemistry. ''JCS'' is currently indexed in: Chemical Abstracts Service (CAS), SCOPUS, EBSCOhost, PubMed, and Web of Science. Anthony Czarnik } Anthony W. Czarnik (born 1957) is an American chemist and inventor. He is best known for pioneering studies in the field of fluorescent Molecular sensor, chemosensors and co-founding Illumina, Inc., a biotechnology company in San Diego. Czarnik ... served the founding editor; he served as Editor from 1999-2010. M.G. Finn served as Editor from 2010-2020. In 2010, ACS agreed to change the name of the journal to ''"Combinatorial Science"'' and it was the first and only ACS journal to be devo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chloromethylation
The Blanc chloromethylation (also called the Blanc reaction) is the chemical reaction of aromatic rings with formaldehyde and hydrogen chloride to form chloromethyl arenes. The reaction is catalyzed by Lewis acids such as zinc chloride. The reaction was discovered by Gustave Louis Blanc (1872-1927) in 1923 Mechanism and scope The reaction is carried out under acidic conditions and with a ZnCl2 catalyst. These conditions protonate the formaldehyde carbonyl making the carbon much more electrophilic. The aldehyde is then attacked by the aromatic pi-electrons, followed by rearomatization of the aromatic ring. The benzyl alcohol thus formed is quickly converted to the chloride under the reaction conditions. Other possibilities for the electrophile include (chloromethyl)oxonium cation (ClH2C–OH2+) or chlorocarbenium cation (ClCH2+), which may be formed in the presence of zinc chloride. These species may account for the fact that moderately and strongly deactivated substrates th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Styrene
Styrene () is an organic compound with the chemical formula C6H5CH=CH2. This derivative of benzene is a colorless oily liquid, although aged samples can appear yellowish. The compound evaporates easily and has a sweet smell, although high concentrations have a less pleasant odor. Styrene is the precursor to polystyrene and several copolymers. Approximately 25 million tonnes of styrene were produced in 2010, increasing to around 35 million tonnes by 2018. Natural occurrence Styrene is named after storax balsam (often commercially sold as ''styrax''), the resin of Liquidambar trees of the Altingiaceae plant family. Styrene occurs naturally in small quantities in some plants and foods (cinnamon, coffee beans, balsam tree (other), balsam trees and peanuts) and is also found in coal tar. History In 1839, the German apothecary Eduard Simon isolated a volatile liquid from the resin (called ''storax'' or ''styrax'' (Latin)) of the Liquidambar styraciflua, American sweetgu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
4-Vinylbenzyl Chloride
4-Vinylbenzyl chloride is an organic compound with the formula ClCH2C6H4CH=CH2. It is a bifunctional molecule, featuring both vinyl and a benzylic chloride functional groups. It is a colorless liquid that is typically stored with a stabilizer to suppress polymerization. In combination with styrene, vinylbenzyl chloride is used as a comonomer in the production of chloromethylated polystyrene.Montheard, Jean Pierre; Jegat, Corinne; Camps, Marcel "Vinylbenzylchloride (chloromethylstyrene), polymers, and copolymers. Recent reactions and applications" Journal of Macromolecular Science, Reviews in Macromolecular Chemistry and Physics 1999, volume C39, pp. 135-174. It is produced by the chlorination of vinyltoluene 4-Vinyltoluene is an organic compound with the formula CH3C6H4CH=CH2. It is derivative of styrene and is used as a comonomer in the production of specialized polystyrenes. It is produced by the dehydrogenation of 4-ethyltoluene. It is also somet .... Often vinyltolu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Copolymers
In polymer chemistry, a copolymer is a polymer derived from more than one species of monomer. The polymerization of monomers into copolymers is called copolymerization. Copolymers obtained from the copolymerization of two monomer species are sometimes called ''bipolymers''. Those obtained from three and four monomers are called ''terpolymers'' and ''quaterpolymers'', respectively. Copolymers can be characterized by a variety of techniques such as NMR spectroscopy and size-exclusion chromatography to determine the molecular size, weight, properties, and composition of the material. Commercial copolymers include acrylonitrile butadiene styrene (ABS), styrene/butadiene co-polymer (SBR), nitrile rubber, styrene-acrylonitrile, styrene-isoprene-styrene (SIS) and ethylene-vinyl acetate, all of which are formed by chain-growth polymerization. Another production mechanism is step-growth polymerization, which is used to produce the nylon-12/6/66 copolymer of nylon 12, nylon 6 and nylo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |