|
Maximum Density
The maximum density of a substance is the highest attainable density of the substance under given conditions. Attaining maximum density Almost all known substances undergo thermal expansion in response to heating, meaning that a given mass of substance contracts to a low volume at low temperatures, when little thermal energy is present. Substances, especially fluids in which intermolecular forces are weak, also undergo compression upon the application of pressure. Nearly all substances therefore reach a density maximum at very low temperatures and very high pressures, characteristic properties of the solid state of matter. Water An especially notable irregular maximum density is that of water, which reaches a density peak at . This has important ramifications in Earth's ecosystem. References See also * List of elements by density * Density * Specific Gravity * Specific weight * Charge density * Buoyancy * Hydrometer A hydrometer or lactometer is an instrument used for ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Substance
A chemical substance is a form of matter having constant chemical composition and characteristic properties. Some references add that chemical substance cannot be separated into its constituent Chemical element, elements by physical separation methods, i.e., without breaking chemical bonds. Chemical substances can be simple substances (substances consisting of a single chemical element), chemical compounds, or alloys. Chemical substances are often called 'pure' to set them apart from mixtures. A common example of a chemical substance is pure Water (molecule), water; it has the same properties and the same atomic ratio, ratio of hydrogen to oxygen whether it is isolated from a river or made in a laboratory. Other chemical substances commonly encountered in pure form are diamond (carbon), gold, Edible salt, table salt (sodium chloride) and refined sugar (sucrose). However, in practice, no substance is entirely pure, and chemical purity is specified according to the intended use of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Water
Water (chemical formula ) is an inorganic, transparent, tasteless, odorless, and nearly colorless chemical substance, which is the main constituent of Earth's hydrosphere and the fluids of all known living organisms (in which it acts as a solvent). It is vital for all known forms of life, despite not providing food, energy or organic micronutrients. Its chemical formula, H2O, indicates that each of its molecules contains one oxygen and two hydrogen atoms, connected by covalent bonds. The hydrogen atoms are attached to the oxygen atom at an angle of 104.45°. "Water" is also the name of the liquid state of H2O at standard temperature and pressure. A number of natural states of water exist. It forms precipitation in the form of rain and aerosols in the form of fog. Clouds consist of suspended droplets of water and ice, its solid state. When finely divided, crystalline ice may precipitate in the form of snow. The gaseous state of water is steam or water vapor. W ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Condensed Matter Physics
Condensed matter physics is the field of physics that deals with the macroscopic and microscopic physical properties of matter, especially the solid and liquid phases which arise from electromagnetic forces between atoms. More generally, the subject deals with "condensed" phases of matter: systems of many constituents with strong interactions between them. More exotic condensed phases include the superconducting phase exhibited by certain materials at low temperature, the ferromagnetic and antiferromagnetic phases of spins on crystal lattices of atoms, and the Bose–Einstein condensate found in ultracold atomic systems. Condensed matter physicists seek to understand the behavior of these phases by experiments to measure various material properties, and by applying the physical laws of quantum mechanics, electromagnetism, statistical mechanics, and other theories to develop mathematical models. The diversity of systems and phenomena available for study makes condensed ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrometer
A hydrometer or lactometer is an instrument used for measuring density or relative density of liquids based on the concept of buoyancy. They are typically calibrated and graduated with one or more scales such as specific gravity. A hydrometer usually consists of a sealed hollow glass tube with a wider bottom portion for buoyancy, a ballast such as lead or mercury for stability, and a narrow stem with graduations for measuring. The liquid to test is poured into a tall container, often a graduated cylinder, and the hydrometer is gently lowered into the liquid until it floats freely. The point at which the surface of the liquid touches the stem of the hydrometer correlates to relative density. Hydrometers can contain any number of scales along the stem corresponding to properties correlating to the density. Hydrometers are calibrated for different uses, such as a lactometer for measuring the density (creaminess) of milk, a saccharometer for measuring the density of sugar in a liq ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
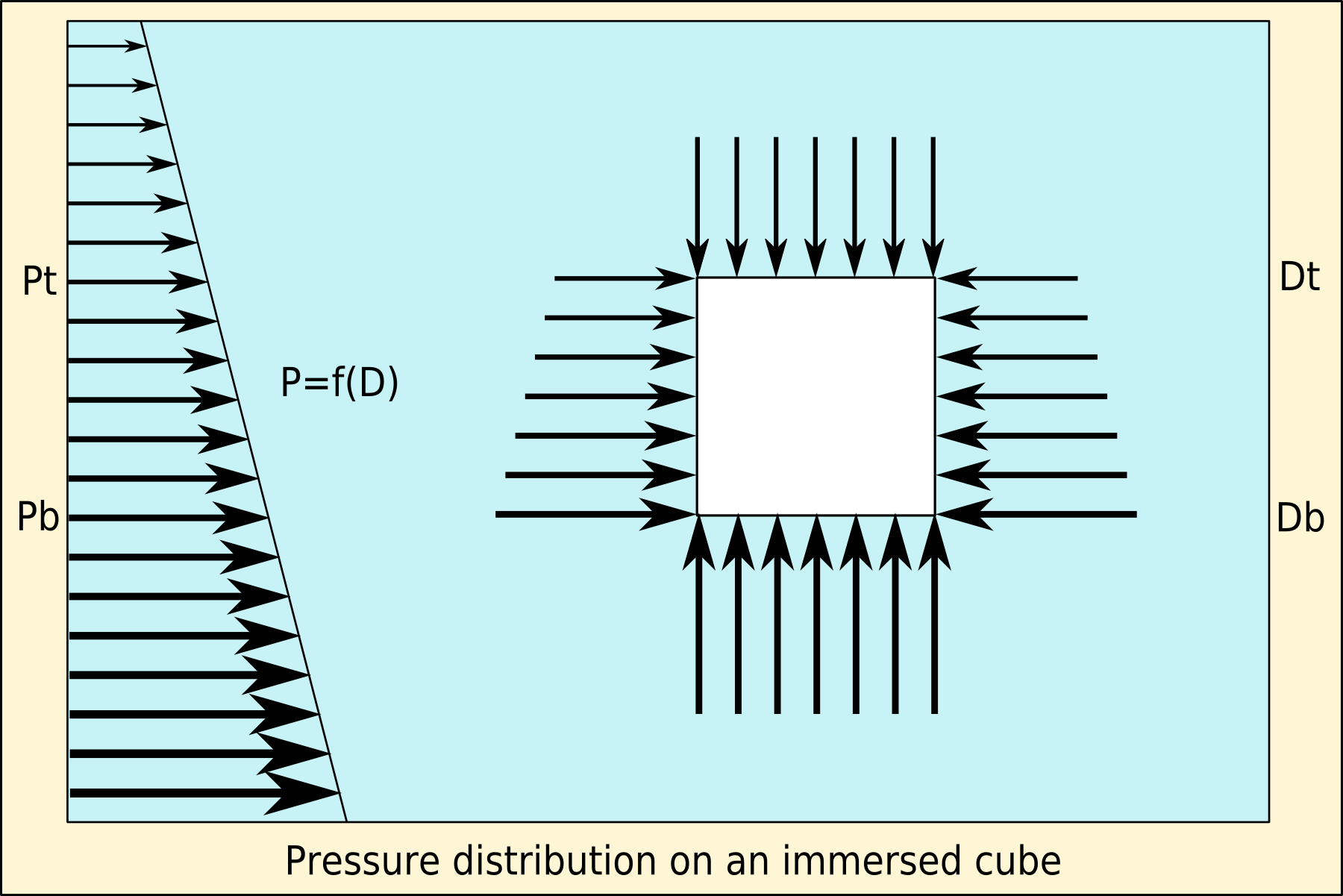
Buoyancy
Buoyancy (), or upthrust, is an upward force exerted by a fluid that opposes the weight of a partially or fully immersed object. In a column of fluid, pressure increases with depth as a result of the weight of the overlying fluid. Thus the pressure at the bottom of a column of fluid is greater than at the top of the column. Similarly, the pressure at the bottom of an object submerged in a fluid is greater than at the top of the object. The pressure difference results in a net upward force on the object. The magnitude of the force is proportional to the pressure difference, and (as explained by Archimedes' principle) is equivalent to the weight of the fluid that would otherwise occupy the submerged volume of the object, i.e. the displaced fluid. For this reason, an object whose average density is greater than that of the fluid in which it is submerged tends to sink. If the object is less dense than the liquid, the force can keep the object afloat. This can occur only in a n ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Charge Density
In electromagnetism, charge density is the amount of electric charge per unit length, surface area, or volume. Volume charge density (symbolized by the Greek letter ρ) is the quantity of charge per unit volume, measured in the SI system in coulombs per cubic meter (C⋅m−3), at any point in a volume. Surface charge density (σ) is the quantity of charge per unit area, measured in coulombs per square meter (C⋅m−2), at any point on a surface charge distribution on a two dimensional surface. Linear charge density (λ) is the quantity of charge per unit length, measured in coulombs per meter (C⋅m−1), at any point on a line charge distribution. Charge density can be either positive or negative, since electric charge can be either positive or negative. Like mass density, charge density can vary with position. In classical electromagnetic theory charge density is idealized as a '' continuous'' scalar function of position \boldsymbol, like a fluid, and \rho(\bol ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Specific Weight
The specific weight, also known as the unit weight, is the weight per unit volume of a material. A commonly used value is the specific weight of water on Earth at , which is .National Council of Examiners for Engineering and Surveying (2005). ''Fundamentals of Engineering Supplied-Reference Handbook'' (7th ed.). . Often a source of confusion is that the terms ''specific gravity'', and less often ''specific weight'', are also used for relative density. A common symbol for specific weight is , the Greek letter Gamma. Definition The specific weight, , of a material is defined as the product of its density, , and the standard gravity, : \gamma = \rho \, g The density of the material is defined as mass per unit volume, typically measured in kg/m3. The standard gravity is acceleration due to gravity, usually given in m/s2, and on Earth usually taken as . Unlike density, specific weight is not a fixed property of a material. It depends on the value of the gravitational acceleration, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Specific Gravity
Relative density, or specific gravity, is the ratio of the density (mass of a unit volume) of a substance to the density of a given reference material. Specific gravity for liquids is nearly always measured with respect to water (molecule), water at its densest (at ); for gases, the reference is air at room temperature (). The term "relative density" (often abbreviated r.d. or RD) is often preferred in scientific usage, whereas the term "specific gravity" is deprecation, deprecated. If a substance's relative density is less than 1 then it is less dense than the reference; if greater than 1 then it is denser than the reference. If the relative density is exactly 1 then the densities are equal; that is, equal volumes of the two substances have the same mass. If the reference material is water, then a substance with a relative density (or specific gravity) less than 1 will float in water. For example, an ice cube, with a relative density of about 0.91, will float. A substance wi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Density
Density (volumetric mass density or specific mass) is the substance's mass per unit of volume. The symbol most often used for density is ''ρ'' (the lower case Greek letter rho), although the Latin letter ''D'' can also be used. Mathematically, density is defined as mass divided by volume: : \rho = \frac where ''ρ'' is the density, ''m'' is the mass, and ''V'' is the volume. In some cases (for instance, in the United States oil and gas industry), density is loosely defined as its weight per unit volume, although this is scientifically inaccurate – this quantity is more specifically called specific weight. For a pure substance the density has the same numerical value as its mass concentration. Different materials usually have different densities, and density may be relevant to buoyancy, purity and packaging. Osmium and iridium are the densest known elements at standard conditions for temperature and pressure. To simplify comparisons of density across different syst ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
List Of Elements By Density
This is a list of the 118 chemical elements which have been identified as of 2022. A chemical element, often simply called an element, is a type of atom which has the same number of protons in its atomic nucleus (i.e., the same atomic number, or ''Z''). The definitive visualisation of all 118 elements is the periodic table of the elements, whose history along the principles of the periodic law was one of the founding developments of modern chemistry. It is a tabular arrangement of the elements by their chemical properties that usually uses abbreviated chemical symbols in place of full element names, but the linear list format presented here is also useful. Like the periodic table, the list below organizes the elements by the number of protons in their atoms; it can also be organized by other properties, such as atomic weight, density, and electronegativity. For more detailed information about the origins of element names, see List of chemical element name etymologies. List See ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ecosystem
An ecosystem (or ecological system) consists of all the organisms and the physical environment with which they interact. These biotic and abiotic components are linked together through nutrient cycles and energy flows. Energy enters the system through photosynthesis and is incorporated into plant tissue. By feeding on plants and on one another, animals play an important role in the movement of matter and energy through the system. They also influence the quantity of plant and microbial biomass present. By breaking down dead organic matter, decomposers release carbon back to the atmosphere and facilitate nutrient cycling by converting nutrients stored in dead biomass back to a form that can be readily used by plants and microbes. Ecosystems are controlled by external and internal factors. External factors such as climate, parent material which forms the soil and topography, control the overall structure of an ecosystem but are not themselves influenced by the ecosyst ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Matter
In classical physics and general chemistry, matter is any substance that has mass and takes up space by having volume. All everyday objects that can be touched are ultimately composed of atoms, which are made up of interacting subatomic particles, and in everyday as well as scientific usage, "matter" generally includes atoms and anything made up of them, and any particles (or combination of particles) that act as if they have both rest mass and volume. However it does not include massless particles such as photons, or other energy phenomena or waves such as light or heat. Matter exists in various states (also known as phases). These include classical everyday phases such as solid, liquid, and gas – for example water exists as ice, liquid water, and gaseous steam – but other states are possible, including plasma, Bose–Einstein condensates, fermionic condensates, and quark–gluon plasma. Usually atoms can be imagined as a nucleus of protons and neutro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |