|
Maribavir
Maribavir, sold under the brand name Livtencity, is an antiviral medication that is used to treat post-transplant cytomegalovirus (CMV). The most common side effects include taste disturbance, nausea, diarrhea, vomiting and fatigue. Maribavir is a cytomegalovirus pUL97 kinase inhibitor that works by preventing the activity of human cytomegalovirus enzyme pUL97, thus blocking virus replication. Maribavir was approved for medical use in the United States in November 2021, and in the European Union in November 2022. Medical uses In the United States, maribavir is indicated to treat people twelve years of age and older and weighing at least with post-transplant cytomegalovirus infection/disease that does not respond (with or without genetic mutations that cause resistance) to available antiviral treatment for cytomegalovirus. In the European Union, maribavir is indicated for the treatment of cytomegalovirus (CMV) infection and/or disease that are refractory (with or without r ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
ViroPharma
ViroPharma Incorporated was a pharmaceutical company that developed and sold drugs that addressed serious diseases treated by physician specialists and in hospital settings. The company focused on product development activities on viruses and human disease, including those caused by cytomegalovirus (CMV) and hepatitis C virus (HCV) infections. It was purchased by Shire in 2013, with Shire paying around $4.2 billion for the company in a deal that was finalized in January 2014. ViroPharma was a member of the NASDAQ Biotechnology Index and the S&P 600. The company had strategic relationships with GlaxoSmithKline, Schering-Plough, and Sanofi-Aventis. ViroPharma acquired Lev Pharmaceuticals in a merger in 2008. History ViroPharma Incorporated was founded in 1994 by Claude H. Nash (Chief Executive Officer), Mark A. McKinlay (Vice President, Research & Development), Marc S. Collett (Vice President, Discovery Research), Johanna A. Griffin (Vice President, Business Development), and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oral Administration
Oral administration is a route of administration where a substance is taken through the mouth. Per os abbreviated to P.O. is sometimes used as a direction for medication to be taken orally. Many medications are taken orally because they are intended to have a systemic effect, reaching different parts of the body via the bloodstream, for example. Oral administration can be easier and less painful than other routes, such as injection. However, the onset of action is relatively low, and the effectiveness is reduced if it is not absorbed properly in the digestive system, or if it is broken down by digestive enzymes before it can reach the bloodstream. Some medications may cause gastrointestinal side effects, such as nausea or vomiting, when taken orally. Oral administration can also only be applied to conscious patients, and patients willing and able to swallow. Terminology ''Per os'' (; ''P.O.'') is an adverbial phrase meaning literally from Latin "through the mouth" or "by mouth ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Enzyme
Enzymes () are proteins that act as biological catalysts by accelerating chemical reactions. The molecules upon which enzymes may act are called substrates, and the enzyme converts the substrates into different molecules known as products. Almost all metabolic processes in the cell need enzyme catalysis in order to occur at rates fast enough to sustain life. Metabolic pathways depend upon enzymes to catalyze individual steps. The study of enzymes is called ''enzymology'' and the field of pseudoenzyme analysis recognizes that during evolution, some enzymes have lost the ability to carry out biological catalysis, which is often reflected in their amino acid sequences and unusual 'pseudocatalytic' properties. Enzymes are known to catalyze more than 5,000 biochemical reaction types. Other biocatalysts are catalytic RNA molecules, called ribozymes. Enzymes' specificity comes from their unique three-dimensional structures. Like all catalysts, enzymes increase the reaction ra ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Committee For Medicinal Products For Human Use
The Committee for Medicinal Products for Human Use (CHMP), formerly known as Committee for Proprietary Medicinal Products (CPMP), is the European Medicines Agency's committee responsible for elaborating the agency's opinions on all issues regarding medicinal products for human use. See also * Committee for Medicinal Products for Veterinary Use The Committee for Medicinal Products for Veterinary Use (CVMP) is the European Medicines Agency's committee responsible for elaborating the agency's opinions on all issues regarding veterinary medicines. Text was copied from this source which is © ... References External links Committee for Medicinal Products for Human Use (CHMP) Health and the European Union {{eu-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Priority Review
Priority review is a program of the United States Food and Drug Administration (FDA) to expedite the review process for drugs that are expected to have a particularly great impact on the treatment of a disease. The priority review voucher program is a program that grants a voucher for priority review to a drug developer as an incentive to develop treatments for disease indications with limited profitability. Priority review vouchers are currently earned by pharmaceutical companies for the development and approval of drugs treating neglected tropical diseases, rare pediatric diseases, and "medical countermeasures" for terrorism. The voucher can be used for future drugs that could have wider indications for use, but the company is required to pay a fee (approximately $2.8 million) to use the voucher. When seeking approval for a drug, manufacturers can apply to the FDA for priority review. This is granted when a drug is intended to treat a serious condition and would "provide a si ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Breakthrough Therapy
Breakthrough therapy is a United States Food and Drug Administration designation that expedites drug development that was created by Congress under Section 902 of the 9 July 2012 Food and Drug Administration Safety and Innovation Act. The FDA's "breakthrough therapy" designation is not intended to imply that a drug is actually a "breakthrough" or that there is high-quality evidence of treatment efficacy for a particular condition; rather, it allows the FDA to grant priority review to drug candidates if preliminary clinical trials indicate that the therapy may offer substantial treatment advantages over existing options for patients with serious or life-threatening diseases. The FDA has other mechanisms for expediting the review and approval process for promising drugs, including fast track designation, accelerated approval, and priority review. Requirements A breakthrough therapy designation can be assigned to a drug if "it is a drug which is intended alone or in combination with ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Orphan Drug
An orphan drug is a pharmaceutical agent developed to treat medical conditions which, because they are so rare, would not be profitable to produce without government assistance. The conditions are referred to as orphan diseases. The assignment of orphan status to a disease and to drugs developed to treat it is a matter of public policy in many countries and has yielded medical breakthroughs that might not otherwise have been achieved, due to the economics of drug research and development. In the U.S. and the EU, it is easier to gain marketing approval for an orphan drug. There may be other financial incentives, such as an extended period of exclusivity, during which the producer has sole rights to market the drug. All are intended to encourage development of drugs which would otherwise lack sufficient profit motive to attract corporate research budgets and personnel. Definition According to the US Food and Drug Administration (FDA), an orphan drug is defined as one "intended for ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Stem Cell
In multicellular organisms, stem cells are undifferentiated or partially differentiated cells that can differentiate into various types of cells and proliferate indefinitely to produce more of the same stem cell. They are the earliest type of cell in a cell lineage. They are found in both embryonic and adult organisms, but they have slightly different properties in each. They are usually distinguished from progenitor cells, which cannot divide indefinitely, and precursor or blast cells, which are usually committed to differentiating into one cell type. In mammals, roughly 50–150 cells make up the inner cell mass during the blastocyst stage of embryonic development, around days 5–14. These have stem-cell capability. ''In vivo'', they eventually differentiate into all of the body's cell types (making them pluripotent). This process starts with the differentiation into the three germ layers – the ectoderm, mesoderm and endoderm – at the gastrulation stage. However ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
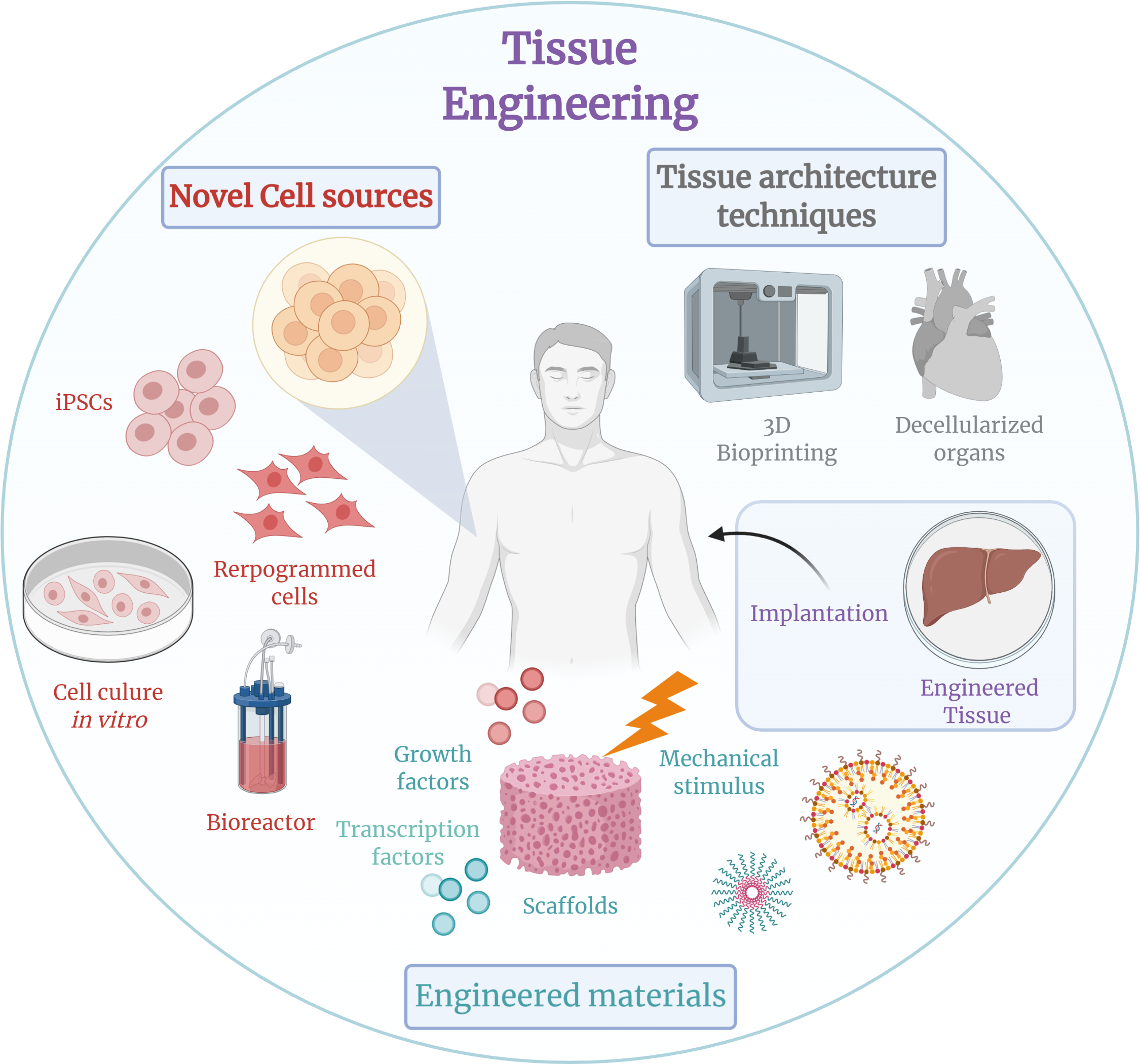
Tissue Engineering
Tissue engineering is a biomedical engineering discipline that uses a combination of Cell (biology), cells, engineering, Materials science, materials methods, and suitable biochemistry, biochemical and physicochemical factors to restore, maintain, improve, or replace different types of biology, biological tissues. Tissue engineering often involves the use of cells placed on tissue scaffolds in the formation of new viable tissue for a medical purpose but is not limited to applications involving cells and tissue scaffolds. While it was once categorized as a sub-field of biomaterials, having grown in scope and importance it can be considered as a field of its own. While most definitions of tissue engineering cover a broad range of applications, in practice the term is closely associated with applications that repair or replace portions of or whole tissues (i.e. bone, Autologous chondrocyte implantation, cartilage, blood vessels, Urinary bladder, bladder, skin, muscle etc.). Often, t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Intent-to-treat
In medicine an intention-to-treat (ITT) analysis of the results of a randomized controlled trial is based on the initial treatment assignment and not on the treatment eventually received. ITT analysis is intended to avoid various misleading artifacts that can arise in intervention research such as non-random attrition of participants from the study or crossover. ITT is also simpler than other forms of study design and analysis, because it does not require observation of compliance status for units assigned to different treatments or incorporation of compliance into the analysis. Although ITT analysis is widely employed in published clinical trials, it can be incorrectly described and there are some issues with its application. Furthermore, there is no consensus on how to carry out an ITT analysis in the presence of missing outcome data. Rationale Randomized clinical trials analyzed by the intention-to-treat (ITT) approach provide unbiased comparisons among the treatment groups. Int ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
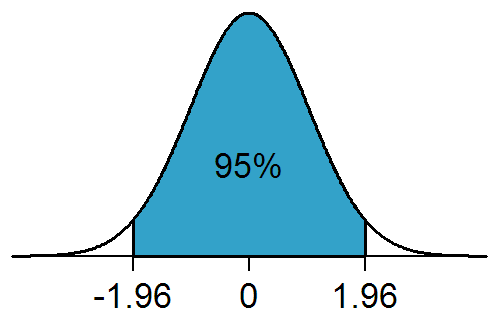
Statistically Significant
In statistical hypothesis testing, a result has statistical significance when it is very unlikely to have occurred given the null hypothesis (simply by chance alone). More precisely, a study's defined significance level, denoted by \alpha, is the probability of the study rejecting the null hypothesis, given that the null hypothesis is true; and the p-value, ''p''-value of a result, ''p'', is the probability of obtaining a result at least as extreme, given that the null hypothesis is true. The result is statistically significant, by the standards of the study, when p \le \alpha. The significance level for a study is chosen before data collection, and is typically set to 5% or much lower—depending on the field of study. In any experiment or Observational study, observation that involves drawing a Sampling (statistics), sample from a Statistical population, population, there is always the possibility that an observed effect would have occurred due to sampling error alone. But if the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Prophylaxis
Preventive healthcare, or prophylaxis, consists of measures taken for the purposes of disease prevention.Hugh R. Leavell and E. Gurney Clark as "the science and art of preventing disease, prolonging life, and promoting physical and mental health and efficiency. Leavell, H. R., & Clark, E. G. (1979). Preventive Medicine for the Doctor in his Community (3rd ed.). Huntington, NY: Robert E. Krieger Publishing Company. Disease and disability are affected by environmental factors, genetic predisposition, disease agents, and lifestyle choices, and are dynamic processes which begin before individuals realize they are affected. Disease prevention relies on anticipatory actions that can be categorized as primal, primary, secondary, and tertiary prevention. Each year, millions of people die of preventable deaths. A 2004 study showed that about half of all deaths in the United States in 2000 were due to preventable behaviors and exposures. Leading causes included cardiovascular disease ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |