|
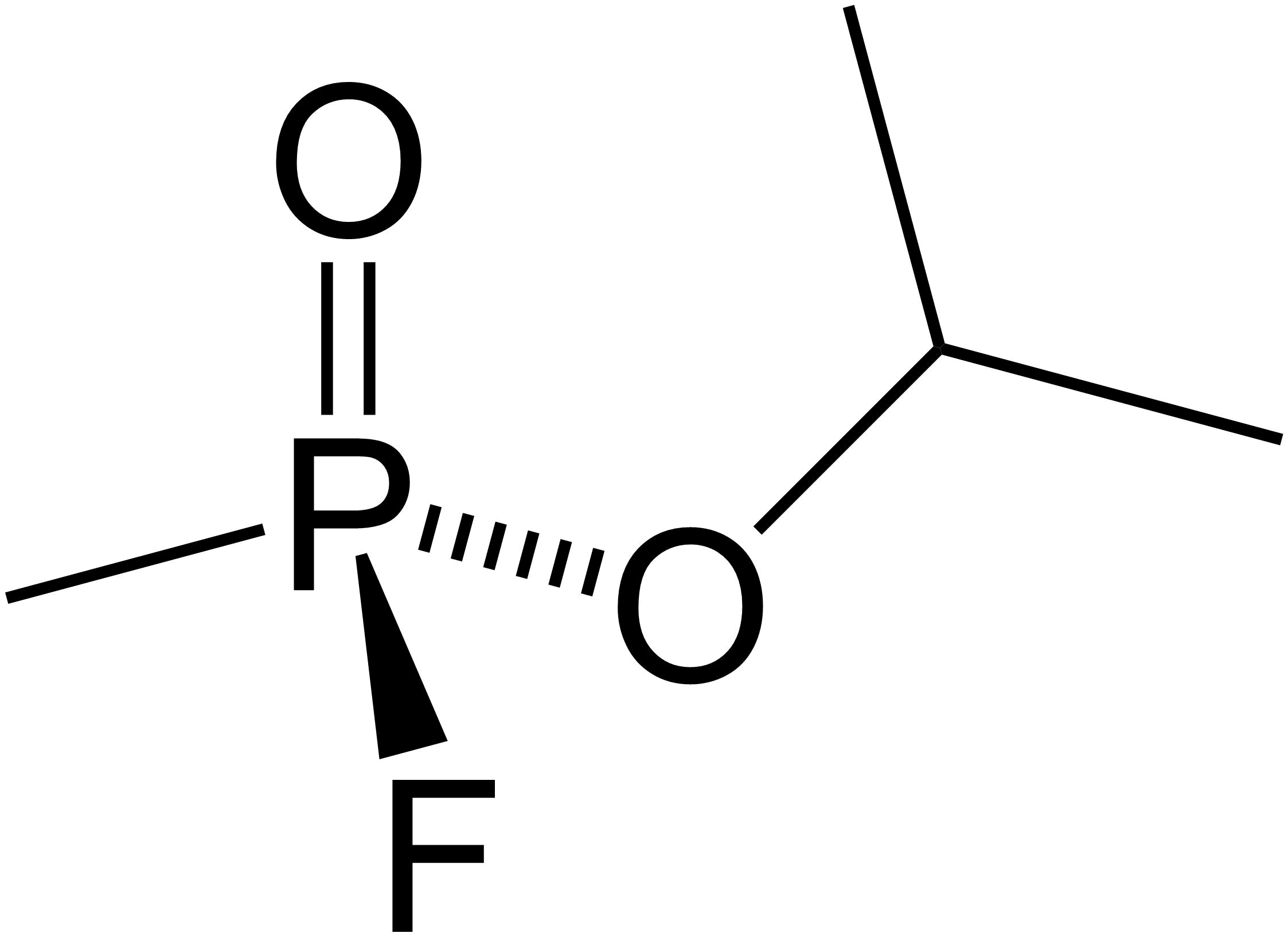
Malaoxon
Malaoxon (Liromat, Malation oxon, Malthon oxon) is a chemical compound with the formula C10H19O7PS. More specifically, it is a phosphorothioate. It is a breakdown product of, and more toxic than, malathion. Air and water This chemical may be sensitive to prolonged exposure to air.National Toxicology Program, 1992 Slightly water-soluble. Fire hazard This chemical is combustible. Health hazard Symptoms of exposure to this type of compound include cholinesterase inhibition, miosis, frontal headache, increased bronchial secretion, nausea, vomiting, sweating, abdominal cramps, diarrhea, lacrimation, increased salivation, bradycardia, cyanosis and muscular twitching of the eyelids, tongue, face and neck, possibly progressing to convulsions. Other symptoms include hyperemia of the conjunctiva, dimness of vision, rhinorrhea, bronchoconstriction, cough, fasciculation, anorexia, incontinence, eye changes, weakness, dyspnea, bronchospasm, hypotension or hypertension due to a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Compound
A chemical compound is a chemical substance composed of many identical molecules (or molecular entities) containing atoms from more than one chemical element held together by chemical bonds. A molecule consisting of atoms of only one element is therefore not a compound. A compound can be transformed into a different substance by a chemical reaction, which may involve interactions with other substances. In this process, bonds between atoms may be broken and/or new bonds formed. There are four major types of compounds, distinguished by how the constituent atoms are bonded together. Molecular compounds are held together by covalent bonds; ionic compounds are held together by ionic bonds; intermetallic compounds are held together by metallic bonds; coordination complexes are held together by coordinate covalent bonds. Non-stoichiometric compounds form a disputed marginal case. A chemical formula specifies the number of atoms of each element in a compound molecule, using the s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Asphyxia
Asphyxia or asphyxiation is a condition of deficient supply of oxygen to the body which arises from abnormal breathing. Asphyxia causes generalized hypoxia, which affects primarily the tissues and organs. There are many circumstances that can induce asphyxia, all of which are characterized by the inability of a person to acquire sufficient oxygen through breathing for an extended period of time. Asphyxia can cause coma or death. In 2015, about 9.8 million cases of unintentional suffocation occurred which resulted in 35,600 deaths. The word asphyxia is from Ancient Greek "without" and , "squeeze" (throb of heart). Causes Situations that can cause asphyxia include but are not limited to: airway obstruction, the constriction or obstruction of airways, such as from asthma, laryngospasm, or simple blockage from the presence of foreign materials; from being in environments where oxygen is not readily accessible: such as underwater, in a low oxygen atmosphere, or in a vacuum; envir ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Reducing Agent
In chemistry, a reducing agent (also known as a reductant, reducer, or electron donor) is a chemical species that "donates" an electron to an (called the , , , or ). Examples of substances that are commonly reducing agents include the Earth metals, formic acid, oxalic acid, and sulfite compounds. In their pre-reaction states, reducers have extra electrons (that is, they are by themselves reduced) and oxidizers lack electrons (that is, they are by themselves oxidized). This is commonly expressed in terms of their oxidation states. An agent's oxidation state describes its degree of loss of electrons, where the higher the oxidation state then the fewer electrons it has. So initially, prior to the reaction, a reducing agent is typically in one of its lower possible oxidation states; its oxidation state increases during the reaction while that of the oxidizer decreases. Thus in a redox reaction, the agent whose oxidation state increases, that "loses/Electron donor, donates electrons ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phosphine Gas
Phosphine (IUPAC name: phosphane) is a colorless, flammable, highly toxic compound with the chemical formula , classed as a pnictogen hydride. Pure phosphine is odorless, but technical grade samples have a highly unpleasant odor like rotting fish, due to the presence of substituted phosphine and diphosphane (). With traces of present, is spontaneously flammable in air (pyrophoric), burning with a luminous flame. Phosphine is a highly toxic respiratory poison, and is immediately dangerous to life or health at 50 ppm. Phosphine has a trigonal pyramidal structure. Phosphines are compounds that include and the organophosphines, which are derived from by substituting one or more hydrogen atoms with organic groups. They have the general formula . Phosphanes are saturated phosphorus hydrides of the form , such as triphosphane. Phosphine, PH3, is the smallest of the phosphines and the smallest of the phosphanes. History Philippe Gengembre (1764–1838), a student of Lavoisier, f ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organophosphate
In organic chemistry, organophosphates (also known as phosphate esters, or OPEs) are a class of organophosphorus compounds with the general structure , a central phosphate molecule with alkyl or aromatic substituents. They can be considered as esters of phosphoric acid. Like most functional groups, organophosphates occur in a diverse range of forms, with important examples including key biomolecules such as DNA, RNA and ATP, as well as many insecticides, herbicides, nerve agents and flame retardants. OPEs have been widely used in various products as flame retardants, plasticizers, and performance additives to engine oil. The popularity of OPEs as flame retardants came as a substitution for the highly regulated brominated flame retardants. The low cost of production and compatibility to diverse polymers made OPEs to be widely used in industry including textile, furniture, electronics as plasticizers and flame retardants. These compounds are added to the final product physica ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ester
In chemistry, an ester is a compound derived from an oxoacid (organic or inorganic) in which at least one hydroxyl group () is replaced by an alkoxy group (), as in the substitution reaction of a carboxylic acid and an alcohol. Glycerides are fatty acid esters of glycerol; they are important in biology, being one of the main classes of lipids and comprising the bulk of animal fats and vegetable oils. Esters typically have a pleasant smell; those of low molecular weight are commonly used as fragrances and are found in essential oils and pheromones. They perform as high-grade solvents for a broad array of plastics, plasticizers, resins, and lacquers, and are one of the largest classes of synthetic lubricants on the commercial market. Polyesters are important plastics, with monomers linked by ester moieties. Phosphoesters form the backbone of DNA molecules. Nitrate esters, such as nitroglycerin, are known for their explosive properties. '' Nomenclature Etymology Th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cholinesterase Inhibitor
Cholinesterase inhibitors (ChEIs), also known as anti-cholinesterase, are chemicals that prevent the breakdown of the neurotransmitter acetylcholine or butyrylcholine. This increases the amount of the acetylcholine or butyrylcholine in the synaptic cleft that can bind to muscarinic receptors, nicotinic receptors and others. This group of inhibitors is divided into two subgroups, acetylcholinesterase inhibitors (AChEIs) and butyrylcholinesterase inhibitors (BChEIs). ChEIs may be used as drugs for Alzheimer's and myasthenia gravis, and also as chemical weapons and insecticides. Side effects when used as drugs may include loss of appetite, nausea, vomiting, loose stools, vivid dreams at night, dehydration, rash, bradycardia, peptic ulcer disease, seizures, weight loss, rhinorrhea, salivation, muscle cramps, and fasciculations. ChEIs are indirect-acting parasympathomimetic drugs. ChEls are widely used as chemical weapons. Since November 2019 the group of ACheIs known as ''Novic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Respiratory Failure
Respiratory failure results from inadequate gas exchange by the respiratory system, meaning that the arterial oxygen, carbon dioxide, or both cannot be kept at normal levels. A drop in the oxygen carried in the blood is known as hypoxemia; a rise in arterial carbon dioxide levels is called hypercapnia. Respiratory failure is classified as either Type 1 or Type 2, based on whether there is a high carbon dioxide level, and can be acute or chronic. In clinical trials, the definition of respiratory failure usually includes increased respiratory rate, abnormal blood gases (hypoxemia, hypercapnia, or both), and evidence of increased work of breathing. Respiratory failure causes an altered mental status due to ischemia in the brain. The typical partial pressure reference values are oxygen Pa more than 80 mmHg (11 kPa) and carbon dioxide Pa less than 45 mmHg (6.0 kPa). Cause Several types of conditions can potentially result in respiratory failure: * Conditions that reduce the fl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Paresthesia
Paresthesia is an abnormal sensation of the skin (tingling, pricking, chilling, burning, numbness) with no apparent physical cause. Paresthesia may be transient or chronic, and may have any of dozens of possible underlying causes. Paresthesias are usually painless and can occur anywhere on the body, but most commonly occur in the arms and legs. The most familiar kind of paresthesia is the sensation known as "pins and needles" after obdormition, having a limb "fall asleep". A less well-known and uncommon paresthesia is formication, the sensation of insects crawling on the skin. Causes Transient Paresthesias of the hands, feet, legs, and arms are common transient symptoms. The briefest electric shock type of paresthesia can be caused by tweaking the ulnar nerve near the elbow; this phenomenon is colloquially known as bumping one's "funny bone". Similar brief shocks can be experienced when any other nerve is tweaked (e.g. a pinched neck nerve may cause a brief shock-like paresthesi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Axonopathy
Polyneuropathy ( poly- + neuro- + -pathy) is damage or disease affecting peripheral nerves (peripheral neuropathy) in roughly the same areas on both sides of the body, featuring weakness, numbness, and burning pain. It usually begins in the hands and feet and may progress to the arms and legs and sometimes to other parts of the body where it may affect the autonomic nervous system. It may be acute or chronic. A number of different disorders may cause polyneuropathy, including diabetes and some types of Guillain–Barré syndrome. Classification Polyneuropathies may be classified in different ways, such as by ''cause'', by ''presentation'', or by ''classes'' of polyneuropathy, in terms of which part of the nerve cell is affected mainly: the axon, the myelin sheath, or the cell body. * ''Distal axonopathy'', is the result of interrupted function of the peripheral nerves. It is the most common response of neurons to metabolic or toxic disturbances, and may be caused by metabolic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
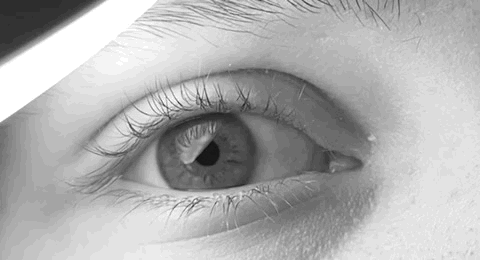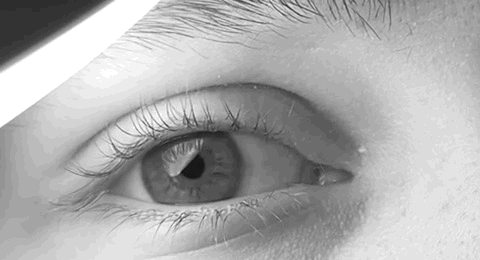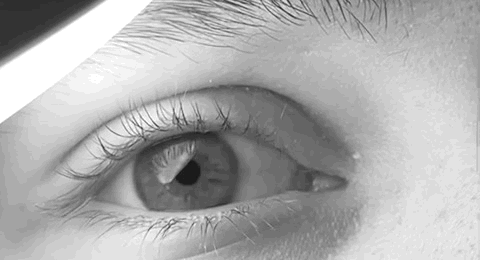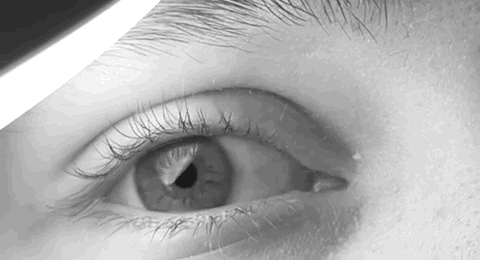
Nystagmus
Nystagmus is a condition of involuntary (or voluntary, in some cases) eye movement. Infants can be born with it but more commonly acquire it in infancy or later in life. In many cases it may result in reduced or limited vision. Due to the involuntary movement of the eye, it has been called "dancing eyes". In normal eyesight, while the head rotates about an axis, distant visual images are sustained by rotating eyes in the opposite direction of the respective axis. The semicircular canals in the vestibule of the ear sense angular acceleration, and send signals to the nuclei for eye movement in the brain. From here, a signal is relayed to the extraocular muscles to allow one's gaze to fix on an object as the head moves. Nystagmus occurs when the semicircular canals are stimulated (e.g., by means of the caloric test, or by disease) while the head is stationary. The direction of ocular movement is related to the semicircular canal that is being stimulated. There are two key form ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cardiac Arrhythmia
Arrhythmias, also known as cardiac arrhythmias, heart arrhythmias, or dysrhythmias, are irregularities in the heartbeat, including when it is too fast or too slow. A resting heart rate that is too fast – above 100 beats per minute in adults – is called tachycardia, and a resting heart rate that is too slow – below 60 beats per minute – is called bradycardia. Some types of arrhythmias have no symptoms. Symptoms, when present, may include palpitations or feeling a pause between heartbeats. In more serious cases, there may be lightheadedness, passing out, shortness of breath or chest pain. While most cases of arrhythmia are not serious, some predispose a person to complications such as stroke or heart failure. Others may result in sudden death. Arrhythmias are often categorized into four groups: extra beats, supraventricular tachycardias, ventricular arrhythmias and bradyarrhythmias. Extra beats include premature atrial contractions, premature ventricular contra ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |

.png)