|
Magnesium Monohydride
Magnesium monohydride is a molecular gas with formula MgH that exists at high temperatures, such as the atmospheres of the Sun and stars. It was originally known as magnesium hydride, although that name is now more commonly used when referring to the similar chemical magnesium dihydride. History George Downing Liveing and James Dewar are claimed to be the first to make and observe a spectral line from MgH in 1878. However they did not realise what the substance was. Formation A laser can evaporate magnesium metal to form atoms that react with molecular hydrogen gas to form MgH and other magnesium hydrides. An electric discharge through hydrogen gas at low pressure (20 pascals) containing pieces of magnesium can produce MgH. Thermally produced hydrogen atoms and magnesium vapour can react and condense in a solid argon matrix. This process does not work with solid neon, probably due to the formation of instead. A simple way to produce some MgH is to burn magnesium in a bunsen b ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Magnesium
Magnesium is a chemical element with the symbol Mg and atomic number 12. It is a shiny gray metal having a low density, low melting point and high chemical reactivity. Like the other alkaline earth metals (group 2 of the periodic table) it occurs naturally only in combination with other elements and it almost always has an oxidation state of +2. It reacts readily with air to form a thin passivation coating of magnesium oxide that inhibits further corrosion of the metal. The free metal burns with a brilliant-white light. The metal is obtained mainly by electrolysis of magnesium salts obtained from brine. It is less dense than aluminium and is used primarily as a component in strong and lightweight alloys that contain aluminium. In the cosmos, magnesium is produced in large, aging stars by the sequential addition of three helium nuclei to a carbon nucleus. When such stars explode as supernovas, much of the magnesium is expelled into the interstellar medium where it ma ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Endothermic
In thermochemistry, an endothermic process () is any thermodynamic process with an increase in the enthalpy (or internal energy ) of the system.Oxtoby, D. W; Gillis, H.P., Butler, L. J. (2015).''Principle of Modern Chemistry'', Brooks Cole. p. 617. In such a process, a closed system usually absorbs thermal energy from its surroundings, which is heat transfer into the system. Thus, an endothermic reaction generally leads to an increase in the temperature of the system and a decrease in that of the surroundings. It may be a chemical process, such as dissolving ammonium nitrate () in water (), or a physical process, such as the melting of ice cubes. The term was coined by 19th-century French chemist Marcellin Berthelot. The opposite of an endothermic process is an exothermic process, one that releases or "gives out" energy, usually in the form of heat and sometimes as electrical energy. Thus in each term (endothermic and exothermic) the prefix refers to where heat (or electrical ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Debye
The debye (symbol: D) (; ) is a CGS unit (a non- SI metric unit) of electric dipole momentTwo equal and opposite charges separated by some distance constitute an electric dipole. This dipole possesses an electric dipole moment whose value is given as charge times length of separation, it is a vector whose direction is in the direction of the unit vector of the position vector of the positive charge w.r.t negative charge: :p = ''q''r. named in honour of the physicist Peter J. W. Debye. It is defined as statcoulomb-centimeters.The statcoulomb is also known as the franklin or electrostatic unit of charge. :1 statC = 1 Fr = 1 esu = 1 cm3/2⋅g1/2⋅s−1. Historically the debye was defined as the dipole moment resulting from two charges of opposite sign but an equal magnitude of 10−10 statcoulomb10−10 statcoulomb corresponds to approximately 0.2083 units of elementary charge. (generally called e.s.u. (electrostatic unit) in older scientific ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Molecular Dipole Moment
In physics, a dipole () is an electromagnetic phenomenon which occurs in two ways: *An electric dipole deals with the separation of the positive and negative electric charges found in any electromagnetic system. A simple example of this system is a pair of charges of equal magnitude but opposite sign separated by some typically small distance. (A permanent electric dipole is called an electret.) *A magnetic dipole is the closed circulation of an electric current system. A simple example is a single loop of wire with constant current through it. A bar magnet is an example of a magnet with a permanent magnetic dipole moment. Dipoles, whether electric or magnetic, can be characterized by their dipole moment, a vector quantity. For the simple electric dipole, the electric dipole moment points from the negative charge towards the positive charge, and has a magnitude equal to the strength of each charge times the separation between the charges. (To be precise: for the definition of th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Covalent
A covalent bond is a chemical bond that involves the sharing of electrons to form electron pairs between atoms. These electron pairs are known as shared pairs or bonding pairs. The stable balance of attractive and repulsive forces between atoms, when they share electrons, is known as covalent bonding. For many molecules, the sharing of electrons allows each atom to attain the equivalent of a full valence shell, corresponding to a stable electronic configuration. In organic chemistry, covalent bonding is much more common than ionic bonding. Covalent bonding also includes many kinds of interactions, including σ-bonding, π-bonding, metal-to-metal bonding, agostic interactions, bent bonds, three-center two-electron bonds and three-center four-electron bonds. The term ''covalent bond'' dates from 1939. The prefix ''co-'' means ''jointly, associated in action, partnered to a lesser degree, '' etc.; thus a "co-valent bond", in essence, means that the atoms share " valence", such a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Moment Of Inertia
The moment of inertia, otherwise known as the mass moment of inertia, angular mass, second moment of mass, or most accurately, rotational inertia, of a rigid body is a quantity that determines the torque needed for a desired angular acceleration about a rotational axis, akin to how mass determines the force needed for a desired acceleration. It depends on the body's mass distribution and the axis chosen, with larger moments requiring more torque to change the body's rate of rotation. It is an extensive (additive) property: for a point mass the moment of inertia is simply the mass times the square of the perpendicular distance to the axis of rotation. The moment of inertia of a rigid composite system is the sum of the moments of inertia of its component subsystems (all taken about the same axis). Its simplest definition is the second moment of mass with respect to distance from an axis. For bodies constrained to rotate in a plane, only their moment of inertia about an axis ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Point Group
In geometry, a point group is a mathematical group of symmetry operations (isometries in a Euclidean space) that have a fixed point in common. The coordinate origin of the Euclidean space is conventionally taken to be a fixed point, and every point group in dimension ''d'' is then a subgroup of the orthogonal group O(''d''). Point groups are used to describe the symmetries of geometric figures and physical objects such as molecules. Each point group can be represented as sets of orthogonal matrices ''M'' that transform point ''x'' into point ''y'' according to Each element of a point group is either a rotation (determinant of ''M'' = 1), or it is a reflection or improper rotation (determinant of ''M'' = −1). The geometric symmetries of crystals are described by space groups, which allow translations and contain point groups as subgroups. Discrete point groups in more than one dimension come in infinite families, but from the crystallographic restriction theorem and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrogen Atom
A hydrogen atom is an atom of the chemical element hydrogen. The electrically neutral atom contains a single positively charged proton and a single negatively charged electron bound to the nucleus by the Coulomb force. Atomic hydrogen constitutes about 75% of the baryonic mass of the universe. In everyday life on Earth, isolated hydrogen atoms (called "atomic hydrogen") are extremely rare. Instead, a hydrogen atom tends to combine with other atoms in compounds, or with another hydrogen atom to form ordinary ( diatomic) hydrogen gas, H2. "Atomic hydrogen" and "hydrogen atom" in ordinary English use have overlapping, yet distinct, meanings. For example, a water molecule contains two hydrogen atoms, but does not contain atomic hydrogen (which would refer to isolated hydrogen atoms). Atomic spectroscopy shows that there is a discrete infinite set of states in which a hydrogen (or any) atom can exist, contrary to the predictions of classical physics. Attempts to develop a theore ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Magnesium
Magnesium is a chemical element with the symbol Mg and atomic number 12. It is a shiny gray metal having a low density, low melting point and high chemical reactivity. Like the other alkaline earth metals (group 2 of the periodic table) it occurs naturally only in combination with other elements and it almost always has an oxidation state of +2. It reacts readily with air to form a thin passivation coating of magnesium oxide that inhibits further corrosion of the metal. The free metal burns with a brilliant-white light. The metal is obtained mainly by electrolysis of magnesium salts obtained from brine. It is less dense than aluminium and is used primarily as a component in strong and lightweight alloys that contain aluminium. In the cosmos, magnesium is produced in large, aging stars by the sequential addition of three helium nuclei to a carbon nucleus. When such stars explode as supernovas, much of the magnesium is expelled into the interstellar medium where it ma ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diatomic Molecule
Diatomic molecules () are molecules composed of only two atoms, of the same or different chemical elements. If a diatomic molecule consists of two atoms of the same element, such as hydrogen () or oxygen (), then it is said to be homonuclear. Otherwise, if a diatomic molecule consists of two different atoms, such as carbon monoxide () or nitric oxide (), the molecule is said to be heteronuclear. The bond in a homonuclear diatomic molecule is non-polar. The only chemical elements that form stable homonuclear diatomic molecules at standard temperature and pressure (STP) (or typical laboratory conditions of 1 bar and 25 °C) are the gases hydrogen (), nitrogen (), oxygen (), fluorine (), and chlorine (). The noble gases (helium, neon, argon, krypton, xenon, and radon) are also gases at STP, but they are monatomic. The homonuclear diatomic gases and noble gases together are called "elemental gases" or "molecular gases", to distinguish them from other gases that are chemical c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sunspot
Sunspots are phenomena on the Sun's photosphere that appear as temporary spots that are darker than the surrounding areas. They are regions of reduced surface temperature caused by concentrations of magnetic flux that inhibit convection. Sunspots appear within active regions, usually in pairs of opposite magnetic polarity. Their number varies according to the approximately 11-year solar cycle. Individual sunspots or groups of sunspots may last anywhere from a few days to a few months, but eventually decay. Sunspots expand and contract as they move across the surface of the Sun, with diameters ranging from to . Larger sunspots can be visible from Earth without the aid of a telescope. They may travel at relative speeds, or proper motions, of a few hundred meters per second when they first emerge. Indicating intense magnetic activity, sunspots accompany other active region phenomena such as coronal loops, prominences, and reconnection events. Most solar flares and coronal mas ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
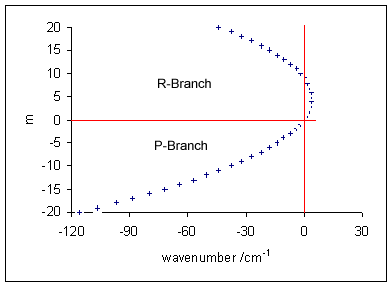
Vibronic Spectroscopy
Vibronic spectroscopy is a branch of molecular spectroscopy concerned with vibronic transitions: the simultaneous changes in electronic and vibrational energy levels of a molecule due to the absorption or emission of a photon of the appropriate energy. In the gas phase, vibronic transitions are accompanied by changes in rotational energy also. Vibronic spectra of diatomic molecules have been analysed in detail; emission spectra are more complicated than absorption spectra. The intensity of allowed vibronic transitions is governed by the Franck–Condon principle. Vibronic spectroscopy may provide information, such as bond length, on electronic excited states of stable molecules. It has also been applied to the study of unstable molecules such as dicarbon, C2, in discharges, flames and astronomical objects.Hollas, p. 211. Principles Electronic transitions are typically observed in the visible and ultraviolet regions, in the wavelength range approximately 200–700 nm ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |




_2007-04-30_T001456.gif)