|
Magnesium Nitride
Magnesium nitride, which possesses the chemical formula Mg3N2, is an inorganic compound of magnesium and nitrogen. At room temperature and pressure it is a greenish yellow powder. Preparation * By passing dry nitrogen over heated magnesium: ::\begin\\ \ce\\ \end * or ammonia: ::\begin\\ \ce\\ \end History When isolating argon, William Ramsay passed dry air over copper to remove oxygen and over magnesium to remove the nitrogen, forming magnesium nitride. Chemistry Magnesium nitride reacts with water to produce magnesium hydroxide and ammonia gas, as do many metal nitrides. :Mg3N2(s) + 6 H2O(l) → 3 Mg(OH)2(aq) + 2 NH3(g) In fact, when magnesium is burned in air, some magnesium nitride is formed in addition to the principal product, magnesium oxide. Thermal decomposition of magnesium nitride gives magnesium and nitrogen gas (at 700-1500 °C). At high pressures, the stability and formation of new nitrogen-rich nitrides (N/Mg ratio equal or greater to one) were sugge ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Beryllium Nitride
Beryllium nitride, Be3N2, is a nitride of beryllium. It can be prepared from the elements at high temperature (1100–1500 °C);Egon Wiberg, Arnold Frederick Holleman (2001) ''Inorganic Chemistry'', Elsevier unlike beryllium azide or BeN6, it decomposes in vacuum into beryllium and nitrogen. It is readily hydrolysed forming beryllium hydroxide and ammonia. It has two polymorphic forms cubic α-Be3N2 with a defect anti-fluorite structure, and hexagonal β-Be3N2. It reacts with silicon nitride, Si3N4 in a stream of ammonia at 1800–1900 °C to form BeSiN2. Preparation Beryllium nitride is prepared by heating beryllium metal powder with dry nitrogen in an oxygen-free atmosphere in temperatures between 700 and 1400 °C. : 3Be + N2 → Be3N2 Uses It is used in refractory ceramicsHugh O. Pierson, 1996, Handbook of Refractory Carbides and Nitrides: Properties, Characteristics, Processing, and Applications, William Andrew Inc., as well as in nuclear reactors and to produ ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nitride
In chemistry, a nitride is an inorganic compound of nitrogen. The "nitride" anion, N3- ion, is very elusive but compounds of nitride are numerous, although rarely naturally occuring. Some nitrides have a find applications, such as wear-resistant coatings (e.g., titanium nitride, TiN), hard ceramic materials (e.g., silicon nitride, Si3N4), and semiconductors (e.g., gallium nitride, GaN). The development of GaN-based light emitting diodes was recognized by the 2014 Nobel Prize in Physics. Metal nitrido complexes are also common. Synthesis of inorganic metal nitrides is challenging because nitrogen gas (N2) is not very reactive at low temperatures, but it becomes more reactive at higher temperatures. Therefore, a balance must be achieved between the low reactivity of nitrogen gas at low temperatures and the entropy driven formation of N2 at high temperatures. However, synthetic methods for nitrides are growing more sophisticated and the materials are of increasing technological re ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
American Institute Of Physics
The American Institute of Physics (AIP) promotes science and the profession of physics, publishes physics journals, and produces publications for scientific and engineering societies. The AIP is made up of various member societies. Its corporate headquarters are at the American Center for Physics in College Park, Maryland, but the institute also has offices in Melville, New York, and Beijing. Historical overview The AIP was founded in 1931 as a response to lack of funding for the sciences during the Great Depression. /www.aip.org/aip/history "History of AIP" American Institute of Physics. July 2010. It formally incorporated in 1932 consisting of five original "member societies", and a total of four thousand members. A new set of member societies was added beginning in the mid-1960s. As soon as the AIP was established it began publishing scientific journals. [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Boron Carbide
Boron carbide (chemical formula approximately B4C) is an extremely hard boron–carbon ceramic, a covalent material used in tank armor, bulletproof vests, engine sabotage powders, as well as numerous industrial applications. With a Vickers hardness of >30 GPa, it is one of the hardest known materials, behind cubic boron nitride and diamond. History Boron carbide was discovered in the 19th century as a by-product of reactions involving metal borides, but its chemical formula was unknown. It was not until the 1930s that the chemical composition was estimated as B4C. Controversy remained as to whether or not the material had this exact 4:1 stoichiometry, as, in practice the material is always slightly carbon-deficient with regard to this formula, and X-ray crystallography shows that its structure is highly complex, with a mixture of C-B-C chains and B12 icosahedra. These features argued against a very simple exact B4C empirical formula. Because of the B12 structural unit, the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diamond
Diamond is a Allotropes of carbon, solid form of the element carbon with its atoms arranged in a crystal structure called diamond cubic. Another solid form of carbon known as graphite is the Chemical stability, chemically stable form of carbon at Standard conditions for temperature and pressure, room temperature and pressure, but diamond is metastable and converts to it at a negligible rate under those conditions. Diamond has the highest Scratch hardness, hardness and thermal conductivity of any natural material, properties that are used in major industrial applications such as cutting and polishing tools. They are also the reason that diamond anvil cells can subject materials to pressures found deep in the Earth. Because the arrangement of atoms in diamond is extremely rigid, few types of impurity can contaminate it (two exceptions are boron and nitrogen). Small numbers of lattice defect, defects or impurities (about one per million of lattice atoms) color diamond blue (bor ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Robert H
The name Robert is an ancient Germanic given name, from Proto-Germanic "fame" and "bright" (''Hrōþiberhtaz''). Compare Old Dutch ''Robrecht'' and Old High German ''Hrodebert'' (a compound of '' Hruod'' ( non, Hróðr) "fame, glory, honour, praise, renown" and ''berht'' "bright, light, shining"). It is the second most frequently used given name of ancient Germanic origin. It is also in use as a surname. Another commonly used form of the name is Rupert. After becoming widely used in Continental Europe it entered England in its Old French form ''Robert'', where an Old English cognate form (''Hrēodbēorht'', ''Hrodberht'', ''Hrēodbēorð'', ''Hrœdbœrð'', ''Hrœdberð'', ''Hrōðberχtŕ'') had existed before the Norman Conquest. The feminine version is Roberta. The Italian, Portuguese, and Spanish form is Roberto. Robert is also a common name in many Germanic languages, including English, German, Dutch, Norwegian, Swedish, Scots, Danish, and Icelandic. It can be use ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
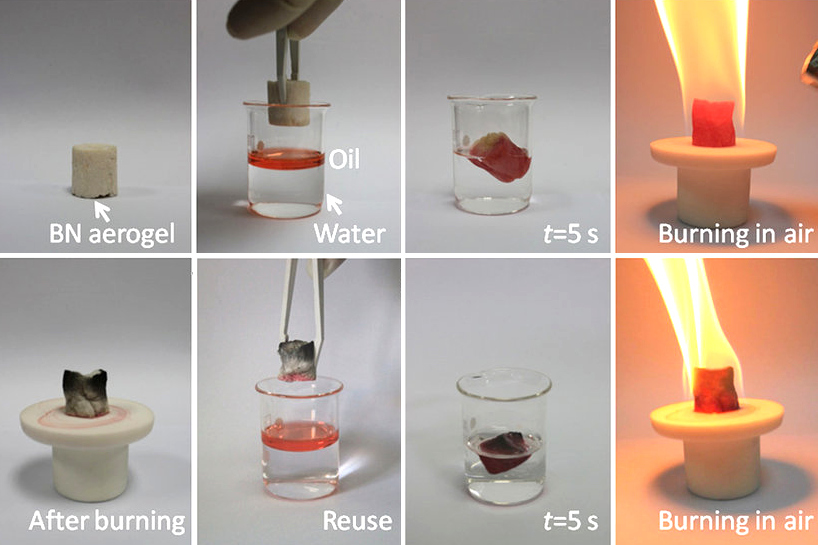
Boron Nitride
Boron nitride is a thermally and chemically resistant refractory compound of boron and nitrogen with the chemical formula BN. It exists in various crystalline forms that are isoelectronic to a similarly structured carbon lattice. The hexagonal form corresponding to graphite is the most stable and soft among BN polymorphs, and is therefore used as a lubricant and an additive to cosmetic products. The cubic ( zincblende aka sphalerite structure) variety analogous to diamond is called c-BN; it is softer than diamond, but its thermal and chemical stability is superior. The rare wurtzite BN modification is similar to lonsdaleite but slightly softer than the cubic form. Because of excellent thermal and chemical stability, boron nitride ceramics are used in high-temperature equipment and metal casting. Boron nitride has potential use in nanotechnology. Structure Boron nitride exists in multiple forms that differ in the arrangement of the boron and nitrogen atoms, giving rise to varyin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Borazon
Borazon is a brand name of a cubic form of boron nitride (cBN). Its color ranges from black to brown and gold, depending on the chemical bond. It is one of the hardest known materials, along with various forms of diamond and kinds of boron nitride. Borazon is a crystal created by heating equal quantities of boron and nitrogen at temperatures greater than 1800 °C (3300 °F) at 7 GPa (1 million lbf/in2). Borazon was first produced in 1957 by Robert H. Wentorf, Jr., a physical chemist working for the General Electric Company. In 1969, General Electric adopted the name Borazon as its trademark for the material. The trademark is now owned by Diamond Innovations, doing business aHyperion Materials & Technologies, Inc. and Borazon is manufactured only by Hyperion Materials & Technologies. Uses and production Borazon has a number of uses , such as: cutting tools, dies, punches, shears, knives, saw blades, bearing rings, needles, rollers, spacers, balls, pump, compres ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Catalyst
Catalysis () is the process of increasing the rate of a chemical reaction by adding a substance known as a catalyst (). Catalysts are not consumed in the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recycles quickly, very small amounts of catalyst often suffice; mixing, surface area, and temperature are important factors in reaction rate. Catalysts generally react with one or more reactants to form intermediates that subsequently give the final reaction product, in the process of regenerating the catalyst. Catalysis may be classified as either homogeneous, whose components are dispersed in the same phase (usually gaseous or liquid) as the reactant, or heterogeneous, whose components are not in the same phase. Enzymes and other biocatalysts are often considered as a third category. Catalysis is ubiquitous in chemical industry of all kinds. Estimates are that 90% of all commercially produced chemical products involve catalysts at some s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Magnesium Oxide
Magnesium oxide ( Mg O), or magnesia, is a white hygroscopic solid mineral that occurs naturally as periclase and is a source of magnesium (see also oxide). It has an empirical formula of MgO and consists of a lattice of Mg2+ ions and O2− ions held together by ionic bonding. Magnesium hydroxide forms in the presence of water (MgO + H2O → Mg(OH)2), but it can be reversed by heating it to remove moisture. Magnesium oxide was historically known as magnesia alba (literally, the white mineral from Magnesia), to differentiate it from ''magnesia negra'', a black mineral containing what is now known as manganese. Related oxides While "magnesium oxide" normally refers to MgO, the compound magnesium peroxide MgO2 is also known. According to evolutionary crystal structure prediction, MgO2 is thermodynamically stable at pressures above 116 GPa (gigapascals), and a semiconducting suboxide Mg3O2 is thermodynamically stable above 500 GPa. Because of its stability, MgO is used as a model sy ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ammonia
Ammonia is an inorganic compound of nitrogen and hydrogen with the formula . A stable binary hydride, and the simplest pnictogen hydride, ammonia is a colourless gas with a distinct pungent smell. Biologically, it is a common nitrogenous waste, particularly among aquatic organisms, and it contributes significantly to the nutritional needs of terrestrial organisms by serving as a precursor to 45% of the world's food and fertilizers. Around 70% of ammonia is used to make fertilisers in various forms and composition, such as urea and Diammonium phosphate. Ammonia in pure form is also applied directly into the soil. Ammonia, either directly or indirectly, is also a building block for the synthesis of many pharmaceutical products and is used in many commercial cleaning products. It is mainly collected by downward displacement of both air and water. Although common in nature—both terrestrially and in the outer planets of the Solar System—and in wide use, ammonia is both caust ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Calcium Nitride
Calcium nitride is the inorganic compound with the chemical formula Ca3 N2. It exists in various forms (isomorphs), α-calcium nitride being more commonly encountered. Structure α-Calcium nitride adopts an anti-bixbyite structure, similar to Mn2O3, except that the positions of the ions are reversed: calcium (Ca2+) take the oxide (O2−) positions and nitride ions (N3−) the manganese (Mn3+). In this structure, Ca2+ occupies tetrahedral sites, and the nitride centres occupy two different types of octahedral sites. Synthesis and reactions Calcium nitride is formed along with the oxide, CaO, when calcium burns in air. It can be produced by direct reaction of the elements: :3 Ca + N2 → Ca3N2 It reacts with water or even the moisture in air to give ammonia and calcium hydroxide: :Ca3N2 + 6 H2O → 3 Ca(OH)2 + 2 NH3 Like sodium oxide Sodium oxide is a chemical compound with the formula Na2 O. It is used in ceramics and glasses. It is a white solid but the c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |


-3D-balls.png)