|
Magnesium (pharmaceutical Preparation)
Magnesium salts are available as a medication in a number of formulations. They are used to treat magnesium deficiency, low blood magnesium, eclampsia, and several other conditions. Magnesium is important to health. Usually in lower dosages, magnesium is commonly included in dietary mineral preparations, including many multivitamin preparations. Chelated magnesium is sometimes used to aid in absorption. In 2020, it was the 202nd most commonly prescribed medication in the United States, with more than 2million prescriptions. Medical uses *As a bronchodilator after beta-agonist and anticholinergic agents have been tried, e.g. in severe exacerbations of asthma. Recent studies have revealed that magnesium sulfate can be nebulized to reduce the symptoms of acute asthma. It is commonly administered via the intravenous route for the management of severe asthma attacks. *Obstetrics: Magnesium sulfate is used to prevent seizures in women with preeclampsia and eclampsia, and is also ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Magnesium Deficiency (medicine)
Magnesium deficiency is an electrolyte disturbance in which there is a low level of magnesium in the body. It can result in multiple symptoms. Symptoms include tremor, poor coordination, muscle spasms, loss of appetite, personality changes, and nystagmus. Complications may include seizures or cardiac arrest such as from torsade de pointes. Those with low magnesium often have low potassium. Causes include low dietary intake, alcoholism, diarrhea, increased urinary loss, malabsorption, poor absorption from the intestines, and diabetes mellitus. A number of medications may also cause low magnesium, including proton pump inhibitors (PPIs) and furosemide. The diagnosis is typically based on finding low blood magnesium levels (hypomagnesemia). Normal magnesium levels are between 0.6 and 1.1 mmol/L (1.46–2.68 mg/dL) with levels less than 0.6 mmol/L (1.46 mg/dL) defining hypomagnesemia. Specific electrocardiogram (ECG) changes may be seen. Treatment is with magn ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Calcium Gluconate
Calcium gluconate is a mineral supplement and medication. As a medication it is used by injection into a vein to treat low blood calcium, high blood potassium, and magnesium toxicity. Supplementation is generally only required when there is not enough calcium in the diet. Supplementation may be done to treat or prevent osteoporosis or rickets. It can also be taken by mouth but is not recommended for injection into a muscle. Side effects when injected include slow heart rate, pain at the site of injection, and low blood pressure. When taken by mouth side effects may include constipation and nausea. Blood calcium levels should be measured when used and extra care should be taken in those with a history of kidney stones. At normal doses, use is regarded as safe in pregnancy and breastfeeding. Calcium gluconate is made by mixing gluconic acid with calcium carbonate or calcium hydroxide. Calcium gluconate came into medical use in the 1920s. It is on the World Health Organization ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Antacid
An antacid is a substance which neutralizes stomach acidity and is used to relieve heartburn, indigestion or an upset stomach. Some antacids have been used in the treatment of constipation and diarrhea. Marketed antacids contain salts of aluminum, calcium, magnesium, or sodium. Some preparations contain a combination of two salts, such as magnesium carbonate and aluminium hydroxide (e.g. hydrotalcite). Medical uses Antacids are available over the counter and are taken by mouth to quickly relieve occasional heartburn, the major symptom of gastroesophageal reflux disease and indigestion. Treatment with antacids alone is symptomatic and only justified for minor symptoms.U.S. Department of Health & Human Services. Agency for Healthcare Research and Quality 23 September 201Consumer Summary – Treatment Options for GERD or Acid Reflux Disease: A Review of the Research for Adults Alternative uses for antacids include constipation, diarrhea, hyperphosphatemia, and urinary alkalizatio ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Magnesium Hydroxide
Magnesium hydroxide is the inorganic compound with the chemical formula Mg(OH)2. It occurs in nature as the mineral brucite. It is a white solid with low solubility in water (). Magnesium hydroxide is a common component of antacids, such as milk of magnesia. Preparation Treating the solution of different soluble magnesium salts with alkaline water induces the precipitation of the solid hydroxide Mg(OH)2: :Mg2+ + 2OH− → Mg(OH)2 As is the second most abundant cation present in seawater after , it can be economically extracted directly from seawater by alkalinisation as described here above. On an industrial scale, Mg(OH)2 is produced by treating seawater with lime (Ca(OH)2). A volume of (or 160,000 US gallons) of seawater gives about one ton of Mg(OH)2. Ca(OH)2 is far more soluble than Mg(OH)2 and drastically increases the pH value of seawater from 8.2 to 12.5. The less soluble precipitates because of the common ion effect due to the added by the dissolution of : : ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Magnesium Citrate
Magnesium citrate is a magnesium preparation in salt form with citric acid in a 1:1 ratio (1 magnesium atom per citrate molecule). The name "magnesium citrate" is ambiguous and sometimes may refer to other salts such as trimagnesium citrate which has a magnesium:citrate ratio of 3:2. Magnesium citrate is used medicinally as a saline laxative and to completely empty the bowel prior to a major surgery or colonoscopy. It is available without a prescription, both as a generic and under various brand names. It is also used in the pill form as a magnesium dietary supplement. It contains 11.23% magnesium by weight. Compared to trimagnesium citrate, it is much more water-soluble, less alkaline, and contains less magnesium. As a food additive, magnesium citrate is used to regulate acidity and is known as E number E345. Mechanism of action Magnesium citrate works by attracting water through the tissues by a process known as osmosis. Once in the intestine, it can attract enough water ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
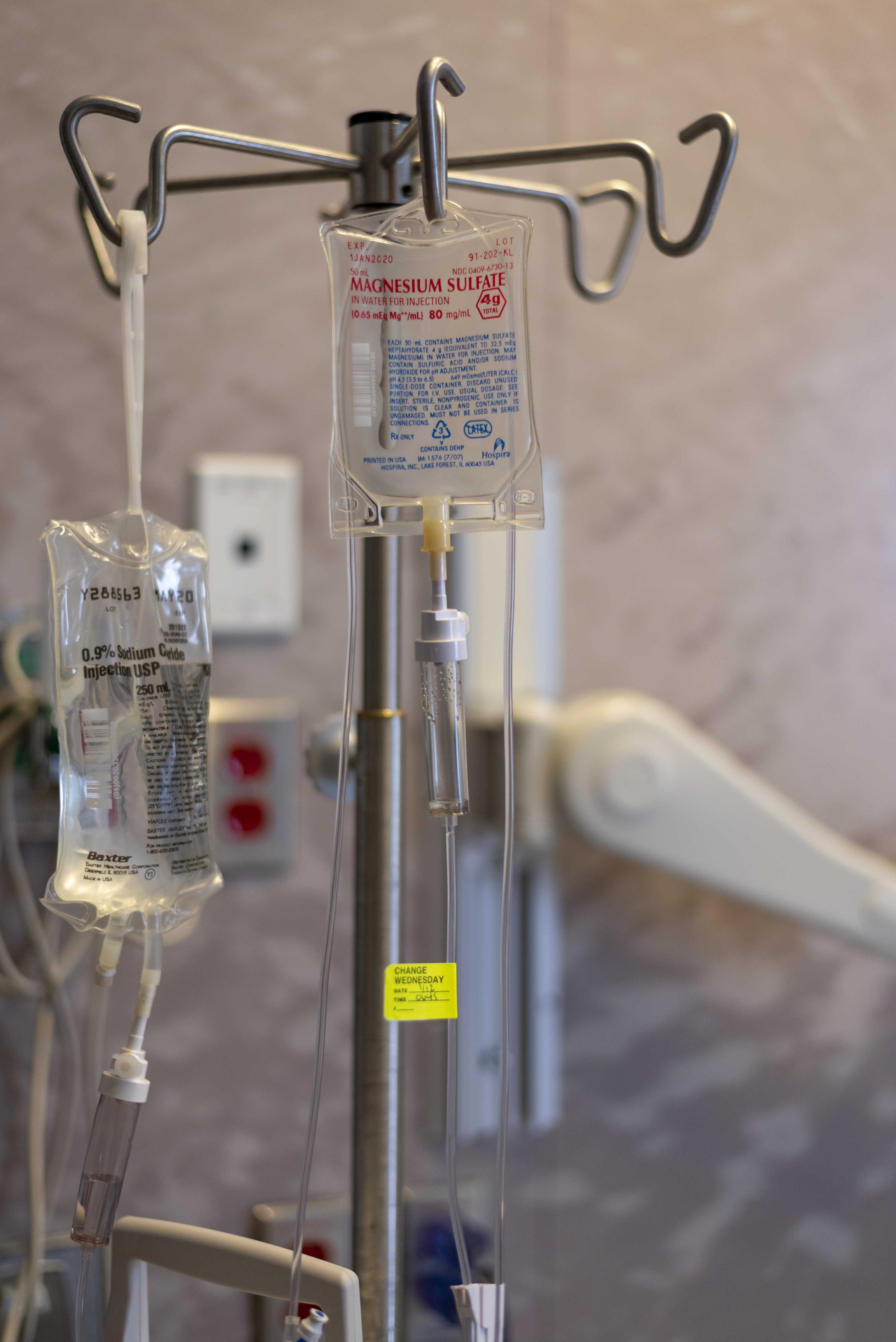
Magnesium Sulfate (medical Use)
Magnesium sulfate as a medication is used to treat and prevent low blood magnesium and seizures in women with eclampsia. It is also used in the treatment of torsades de pointes, severe asthma exacerbations, constipation, and barium poisoning. It is given by injection into a vein or muscle as well as by mouth. As epsom salts, it is also used for mineral baths. Common side effects include low blood pressure, skin flushing, and low blood calcium. Other side effects may include vomiting, muscle weakness, and decreased breathing. While there is evidence that use during pregnancy may harm the baby, the benefits in certain conditions are greater than the risks. Its use during breastfeeding is deemed to be safe. The way it works is not fully understood, but is believed to involve depressing the action of neurons. Magnesium sulfate came into medical use at least as early as 1618. It is on the World Health Organization's List of Essential Medicines. Forms Magnesium sulfate is ava ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Magnesium Oxide
Magnesium oxide ( Mg O), or magnesia, is a white hygroscopic solid mineral that occurs naturally as periclase and is a source of magnesium (see also oxide). It has an empirical formula of MgO and consists of a lattice of Mg2+ ions and O2− ions held together by ionic bonding. Magnesium hydroxide forms in the presence of water (MgO + H2O → Mg(OH)2), but it can be reversed by heating it to remove moisture. Magnesium oxide was historically known as magnesia alba (literally, the white mineral from Magnesia), to differentiate it from ''magnesia negra'', a black mineral containing what is now known as manganese. Related oxides While "magnesium oxide" normally refers to MgO, the compound magnesium peroxide MgO2 is also known. According to evolutionary crystal structure prediction, MgO2 is thermodynamically stable at pressures above 116 GPa (gigapascals), and a semiconducting suboxide Mg3O2 is thermodynamically stable above 500 GPa. Because of its stability, MgO is used as a model sy ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Magnesium Orotate
Magnesium orotate, the magnesium salt of orotic acid, is a mineral supplement. It can be used in treating extracellular magnesium deficiency, as well as in mitigating magnesium depletion that inhibits the binding of adenosine triphosphate Adenosine triphosphate (ATP) is an organic compound that provides energy to drive many processes in living cells, such as muscle contraction, nerve impulse propagation, condensate dissolution, and chemical synthesis. Found in all known forms of ... via orotic acid, which provides binding sites. References Magnesium compounds {{gastrointestinal-drug-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Magnesium Lactate
Magnesium lactate, the magnesium salt of lactic acid, is a mineral supplement In the context of nutrition, a mineral is a chemical element required as an essential nutrient by organisms to perform functions necessary for life. However, the four major structural elements in the human body by weight (oxygen, hydrogen, carbon .... Added to some food and beverages as an acidity regulator and labeled as E329. Magnesium compounds Lactates {{gastrointestinal-drug-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Magnesium Glycinate
Magnesium glycinate, also known as magnesium diglycinate or magnesium bisglycinate, is the magnesium salt of glycine (one magnesium and two glycine molecules), and is sold as a dietary supplement. It contains 14.1% elemental magnesium by mass. Accordingly, 141 mg of elemental magnesium is contained in 1000 mg of magnesium glycinate. Uses Magnesium glycinate has been studied with applicability to patients with a bowel resection or pregnancy-induced leg cramps. See also * Magnesium (pharmaceutical preparation) * Magnesium deficiency (medicine) * Magnesium in biology Magnesium is an essential element in biological systems. Magnesium occurs typically as the Mg2+ ion. It is an essential mineral nutrient (i.e., element) for life and is present in every cell type in every organism. For example, adenosine triphosph ... References {{organic-compound-stub Magnesium compounds Glycinates Dietary supplements ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Magnesium Gluconate
Magnesium gluconate is a compound with formula MgC12H22O14. It is the magnesium salt of gluconic acid. According to one study, magnesium gluconate showed the highest level of bioavailability of any magnesium salt which implies its viability as a supplement, although of the 10 salts studied, all increased magnesium levels significantly. This study did not include magnesium glycinate, which is one of the most bioavailable forms of magnesium. It has E number "E580". Use in medicine There are data on the pharmacological properties of magnesium gluconate. Gluconic acid is the initial substrate for the reactions of pentose phosphate path of oxidation of glucose, so it was suggested that it may affect the energy metabolism of mitochondria. In Ukraine, magnesium gluconate, together with potassium gluconate Potassium gluconate is the potassium salt of the conjugate base of gluconic acid. It is also referred to as 2,3,4,5,6-pentahydroxycaproic acid potassium salt, D-gluconic acid potas ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Magnesium Chloride
Magnesium chloride is the family of inorganic compounds with the formula , where x can range from 0 to 12. These salts are colorless or white solids that are highly soluble in water. These compounds and their solutions, both of which occur in nature, have a variety of practical uses. Anhydrous magnesium chloride is the principal precursor to magnesium metal, which is produced on a large scale. Hydrated magnesium chloride is the form most readily available. Production Magnesium chloride can be extracted from brine or sea water. In North America, it is produced primarily from Great Salt Lake brine. In the Jordan Valley, it is obtained from the Dead Sea. The mineral bischofite () is extracted (by solution mining) out of ancient seabeds, for example, the Zechstein seabed in northwest Europe. Some deposits result from high content of magnesium chloride in the primordial ocean. Some magnesium chloride is made from evaporation of seawater. In the Dow process, magnesium chloride is regen ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |