|
MTOR Inhibitors
mTOR inhibitors are a class of drugs that inhibit the mechanistic target of rapamycin (mTOR), which is a serine/threonine-specific protein kinase that belongs to the family of phosphatidylinositol-3 kinase (PI3K) related kinases (PIKKs). mTOR regulates cellular metabolism, growth, and proliferation by forming and signaling through two protein complexes, mTORC1 and mTORC2. The most established mTOR inhibitors are so-called rapalogs (rapamycin and its analogs), which have shown tumor responses in clinical trials against various tumor types. History The discovery of mTOR was made a few decades ago while investigating the mechanism of action of its inhibitor, rapamycin. Rapamycin was first discovered in 1975 in a soil sample from Easter Island of South Pacific, also known as Rapa Nui, from where its name is derived. Rapamycin is a macrolide, produced by the microorganism ''Streptomyces hygroscopicus'' and showed antifungal properties. Shortly after its discovery, immunosuppressive ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Immunosuppression
Immunosuppression is a reduction of the activation or efficacy of the immune system. Some portions of the immune system itself have immunosuppressive effects on other parts of the immune system, and immunosuppression may occur as an adverse reaction to treatment of other conditions. In general, deliberately induced immunosuppression is performed to prevent the body from rejecting an organ transplant. Additionally, it is used for treating graft-versus-host disease after a bone marrow transplant, or for the treatment of auto-immune diseases such as systemic lupus erythematosus, rheumatoid arthritis, Sjögren's syndrome, or Crohn's disease. This is typically done using medications, but may involve surgery ( splenectomy), plasmapheresis, or radiation. A person who is undergoing immunosuppression, or whose immune system is weak for some other reasons (such as chemotherapy or HIV), is said to be '' immunocompromised''. Deliberately induced Administration of immunosuppressive ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Streptomyces Hygroscopicus
''Streptomyces hygroscopicus'' is a bacterial species in the genus ''Streptomyces''. It was first described by Hans Laurits Jensen in 1931. Biochemistry Cultures of different strains of ''S. hygroscopicus'' can be used to produce a number of chemical compounds or enzymes. Small molecules Immunosuppressants Sirolimus (also known as rapamycin) is an immunosuppressant that has been isolated from ''S. hygroscopicus'' from soil samples from Easter Island. Ascomycin can be used to treat autoimmune diseases and skin diseases, and can help prevent rejection after an organ transplant. Antibiotics The antibiotics geldanamycin, hygromycin B, nigericin, validamycin, and cyclothiazomycin are found in ''S. hygroscopicus''. Experimental cancer drugs Indolocarbazoles can be found in ''S. hygroscopicus'' . Anthelmintics and insecticides Milbemycin and milbemycin oxime can be found in ''S. hygroscopicus'' cultures. Herbicide ''S. hygroscopicus'' also produces the natur ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
C-terminus
The C-terminus (also known as the carboxyl-terminus, carboxy-terminus, C-terminal tail, C-terminal end, or COOH-terminus) is the end of an amino acid chain (protein or polypeptide), terminated by a free carboxyl group (-COOH). When the protein is translated from messenger RNA, it is created from N-terminus to C-terminus. The convention for writing peptide sequences is to put the C-terminal end on the right and write the sequence from N- to C-terminus. Chemistry Each amino acid has a carboxyl group and an amine group. Amino acids link to one another to form a chain by a dehydration reaction which joins the amine group of one amino acid to the carboxyl group of the next. Thus polypeptide chains have an end with an unbound carboxyl group, the C-terminus, and an end with an unbound amine group, the N-terminus. Proteins are naturally synthesized starting from the N-terminus and ending at the C-terminus. Function C-terminal retention signals While the N-terminus of a protein often co ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
N-terminus
The N-terminus (also known as the amino-terminus, NH2-terminus, N-terminal end or amine-terminus) is the start of a protein or polypeptide, referring to the free amine group (-NH2) located at the end of a polypeptide. Within a peptide, the amine group is bonded to the carboxylic group of another amino acid, making it a chain. That leaves a free carboxylic group at one end of the peptide, called the C-terminus, and a free amine group on the other end called the N-terminus. By convention, peptide sequences are written N-terminus to C-terminus, left to right (in LTR writing systems). This correlates the translation direction to the text direction, because when a protein is translated from messenger RNA, it is created from the N-terminus to the C-terminus, as amino acids are added to the carboxyl end of the protein. Chemistry Each amino acid has an amine group and a carboxylic group. Amino acids link to one another by peptide bonds which form through a dehydration reaction ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Domain Structure Of MTOR
Domain may refer to: Mathematics *Domain of a function, the set of input values for which the (total) function is defined ** Domain of definition of a partial function **Natural domain of a partial function **Domain of holomorphy of a function *Domain (mathematical analysis), an open connected set *Domain of discourse, the set of entities over which logic variables may range * Domain of an algebraic structure, the set on which the algebraic structure is defined *Domain theory, the study of certain subsets of continuous lattices that provided the first denotational semantics of the lambda calculus *Domain (ring theory), a nontrivial ring without left or right zero divisors **Integral domain, a non-trivial commutative ring without zero divisors *** Atomic domain, an integral domain in which every non-zero non-unit is a finite product of irreducible elements ***Bézout domain, an integral domain in which the sum of two principal ideals is again a principal ideal ***Euclidean domain, an ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phosphatidylinositol 3-kinase-related Kinase
Phosphatidylinositol 3-kinase-related kinases (PIKKs) are a family of Ser/Thr-protein kinases with sequence similarity to phosphatidylinositol-3 kinases ( PI3Ks). Members The human PIKK family includes six members: Structure PIKKs proteins contain the following four domains: # N-terminus FRAP-ATM- TRRAP (FAT) domain, # kinase domain (KD; PI3_PI4_kinase), # PIKK- regulatory domain (PRD), and # C-terminus The C-terminus (also known as the carboxyl-terminus, carboxy-terminus, C-terminal tail, C-terminal end, or COOH-terminus) is the end of an amino acid chain (protein or polypeptide), terminated by a free carboxyl group (-COOH). When the protein i ... FAT-C-terminal (FATC) domain References External links Kinase Family PIKKaWikiKinome EC 2.7.11 Protein families {{protein-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dual-specificity Kinase
In biochemistry, a dual-specificity kinase () is a kinase that can act as both tyrosine kinase and Serine/threonine-specific protein kinase, serine/threonine kinase. Mitogen-activated protein kinase kinase, MEKs, involved in Mitogen-activated protein kinases, MAP pathways, are principal examples of dual-specificity kinases. Other common examples include: * ADK1 (Arabidopsis dual specificity kinase 1) * CLK1, CLK2, CLK3 (gene), CLK3, CLK4 (gene), CLK4 * DSTYK * DYRK1A, DYRK1B, DYRK2, DYRK3, DYRK4 * Mps1p * TESK1, TESK2 * TTK (gene), TTK The List of enzymes, systematic name of this enzyme class is ATP:protein phosphotransferase (Ser/Thr- and Tyr-phosphorylating). References * * * * * EC 2.7.12 Enzymes of unknown structure {{2.7-enzyme-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Serine/threonine-specific Protein Kinase
A serine/threonine protein kinase () is a kinase enzyme, in particular a protein kinase, that phosphorylates the OH group of the amino-acid residues serine or threonine, which have similar side chains. At least 350 of the 500+ human protein kinases are serine/threonine kinases (STK). In enzymology, the term ''serine/threonine protein kinase'' describes a class of enzymes in the family of transferases, that transfer phosphates to the oxygen atom of a serine or threonine side chain in proteins. This process is called phosphorylation. Protein phosphorylation in particular plays a significant role in a wide range of cellular processes and is a very important posttranslational modification. The chemical reaction performed by these enzymes can be written as :ATP + a protein \rightleftharpoons ADP + a phosphoprotein Thus, the two substrates of this enzyme are ATP and a protein, whereas its two products are ADP and phosphoprotein. The systematic name of this enzyme clas ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
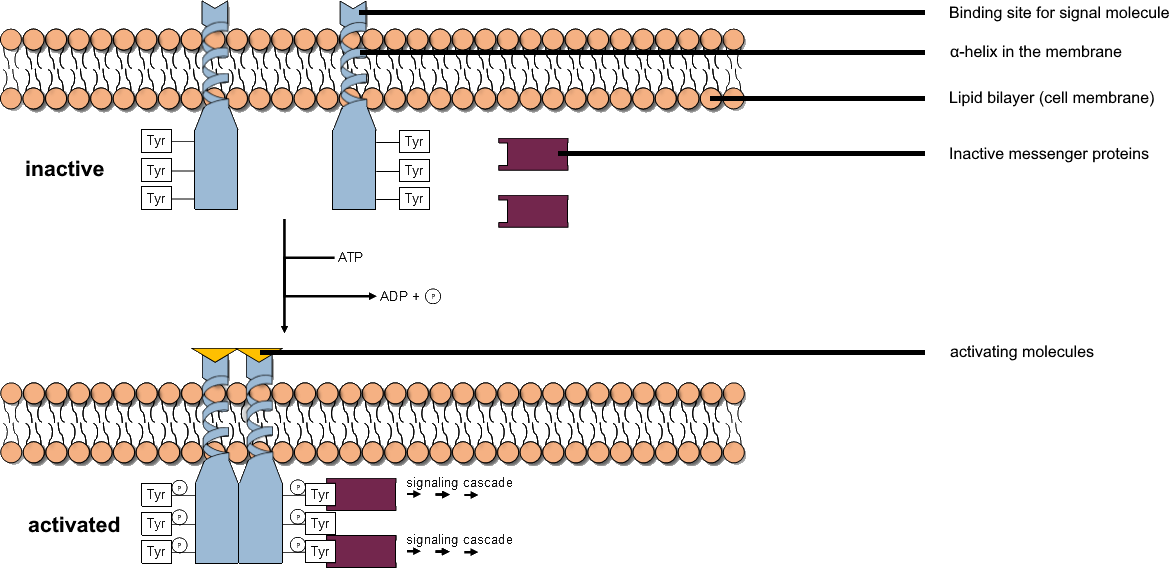
Tyrosine Kinase
A tyrosine kinase is an enzyme that can transfer a phosphate group from ATP to the tyrosine residues of specific proteins inside a cell. It functions as an "on" or "off" switch in many cellular functions. Tyrosine kinases belong to a larger class of enzymes known as protein kinases which also attach phosphates to other amino acids such as serine and threonine. Phosphorylation of proteins by kinases is an important mechanism for communicating signals within a cell (signal transduction) and regulating cellular activity, such as cell division. Protein kinases can become mutated, stuck in the "on" position, and cause unregulated growth of the cell, which is a necessary step for the development of cancer. Therefore, kinase inhibitors, such as imatinib and osimertinib, are often effective cancer treatments. Most tyrosine kinases have an associated protein tyrosine phosphatase, which removes the phosphate group. Reaction Protein kinases are a group of enzymes that possess a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protein Kinase
A protein kinase is a kinase which selectively modifies other proteins by covalently adding phosphates to them (phosphorylation) as opposed to kinases which modify lipids, carbohydrates, or other molecules. Phosphorylation usually results in a functional change of the target protein (substrate) by changing enzyme activity, cellular location, or association with other proteins. The human genome contains about 500 protein kinase genes and they constitute about 2% of all human genes. There are two main types of protein kinase. The great majority are serine/threonine kinases, which phosphorylate the hydroxyl groups of serines and threonines in their targets and most of the others are tyrosine kinases, although additional types exist. Protein kinases are also found in bacteria and plants. Up to 30% of all human proteins may be modified by kinase activity, and kinases are known to regulate the majority of cellular pathways, especially those involved in signal transduction. Chemical ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cell Cycle
The cell cycle, or cell-division cycle, is the series of events that take place in a cell that cause it to divide into two daughter cells. These events include the duplication of its DNA ( DNA replication) and some of its organelles, and subsequently the partitioning of its cytoplasm, chromosomes and other components into two daughter cells in a process called cell division. In cells with nuclei (eukaryotes, i.e., animal, plant, fungal, and protist cells), the cell cycle is divided into two main stages: interphase and the mitotic (M) phase (including mitosis and cytokinesis). During interphase, the cell grows, accumulating nutrients needed for mitosis, and replicates its DNA and some of its organelles. During the mitotic phase, the replicated chromosomes, organelles, and cytoplasm separate into two new daughter cells. To ensure the proper replication of cellular components and division, there are control mechanisms known as cell cycle checkpoints after each of the ke ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |