|
MTAP
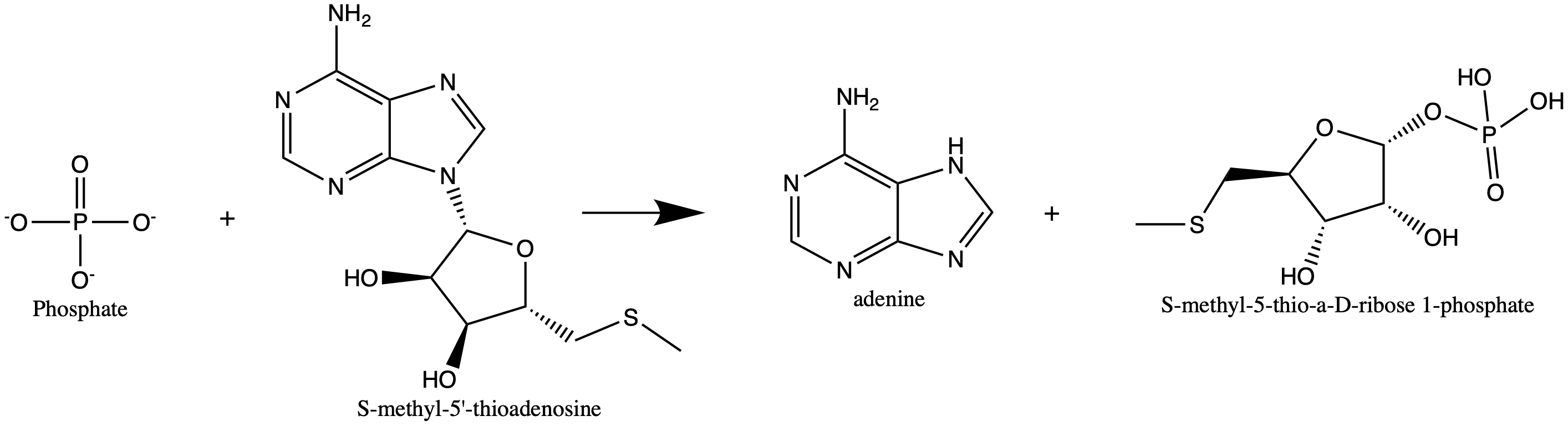
S-methyl-5'-thioadenosine phosphorylase (MTAP) is an enzyme in humans responsible for polyamine metabolism. It is encoded by the methylthioadenosine phosphorylase (MTAP) gene on chromosome 9. Multiple alternatively spliced transcript variants have been described for this gene, but their full-length natures remain unknown. This gene encodes an enzyme that plays a major role in polyamine metabolism and is important for the salvage of both adenine and methionine. It is responsible for the first step in this pathway, where it catalyzes the reversible phosphorylation of MTA to adenine and 5-methylthioribose-1-phosphate. This takes place after MTA is generated from S-adenosylmethionine. MTAP was identified for the first time and characterized likely as a phosphorylase in 1969 by Pegg and Williams-Ashman. The first purification that allowed characterization was by a group in 1986. This purification allowed researchers to investigate why there is the lower expression of MTAP in some ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
MTAP Reaction Summary
S-methyl-5'-thioadenosine phosphorylase (MTAP) is an enzyme in humans responsible for polyamine metabolism. It is encoded by the methylthioadenosine phosphorylase (MTAP) gene on chromosome 9. Multiple alternatively spliced transcript variants have been described for this gene, but their full-length natures remain unknown. This gene encodes an enzyme that plays a major role in polyamine metabolism and is important for the salvage of both adenine and methionine. It is responsible for the first step in this pathway, where it catalyzes the reversible phosphorylation of MTA to adenine and 5-methylthioribose-1-phosphate. This takes place after MTA is generated from S-adenosylmethionine. MTAP was identified for the first time and characterized likely as a phosphorylase in 1969 by Pegg and Williams-Ashman. The first purification that allowed characterization was by a group in 1986. This purification allowed researchers to investigate why there is the lower expression of MTAP in some ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
MTAP Secondary Subunit Structure
S-methyl-5'-thioadenosine phosphorylase (MTAP) is an enzyme in humans responsible for polyamine metabolism. It is encoded by the methylthioadenosine phosphorylase (MTAP) gene on chromosome 9. Multiple alternatively spliced transcript variants have been described for this gene, but their full-length natures remain unknown. This gene encodes an enzyme that plays a major role in polyamine metabolism and is important for the salvage of both adenine and methionine. It is responsible for the first step in this pathway, where it catalyzes the reversible phosphorylation of MTA to adenine and 5-methylthioribose-1-phosphate. This takes place after MTA is generated from S-adenosylmethionine. MTAP was identified for the first time and characterized likely as a phosphorylase in 1969 by Pegg and Williams-Ashman. The first purification that allowed characterization was by a group in 1986. This purification allowed researchers to investigate why there is the lower expression of MTAP in some ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
MTAP Quaternary Structure
S-methyl-5'-thioadenosine phosphorylase (MTAP) is an enzyme in humans responsible for polyamine metabolism. It is encoded by the methylthioadenosine phosphorylase (MTAP) gene on chromosome 9. Multiple alternatively spliced transcript variants have been described for this gene, but their full-length natures remain unknown. This gene encodes an enzyme that plays a major role in polyamine metabolism and is important for the salvage of both adenine and methionine. It is responsible for the first step in this pathway, where it catalyzes the reversible phosphorylation of MTA to adenine and 5-methylthioribose-1-phosphate. This takes place after MTA is generated from S-adenosylmethionine. MTAP was identified for the first time and characterized likely as a phosphorylase in 1969 by Pegg and Williams-Ashman. The first purification that allowed characterization was by a group in 1986. This purification allowed researchers to investigate why there is the lower expression of MTAP in some ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chromosome 9
Chromosome 9 is one of the 23 pairs of chromosomes in humans. Humans normally have two copies of this chromosome, as they normally do with all chromosomes. Chromosome 9 spans about 138 million base pairs of nucleic acids (the building blocks of DNA) and represents between 4.0 and 4.5% of the total DNA in cells. Genes Number of genes These are some of the gene count estimates of human chromosome 9. Because researchers use different approaches to genome annotation, their predictions of the number of genes on each chromosome varies (for technical details, see gene prediction). Among various projects, the collaborative consensus coding sequence project ( CCDS) takes an extremely conservative strategy. So CCDS's gene number prediction represents a lower bound on the total number of human protein-coding genes. Gene list The following is a partial list of genes on human chromosome 9. For a complete list, see the link in the infobox on the right. Diseases and disorders The follow ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Enzyme
Enzymes () are proteins that act as biological catalysts by accelerating chemical reactions. The molecules upon which enzymes may act are called substrates, and the enzyme converts the substrates into different molecules known as products. Almost all metabolic processes in the cell need enzyme catalysis in order to occur at rates fast enough to sustain life. Metabolic pathways depend upon enzymes to catalyze individual steps. The study of enzymes is called ''enzymology'' and the field of pseudoenzyme analysis recognizes that during evolution, some enzymes have lost the ability to carry out biological catalysis, which is often reflected in their amino acid sequences and unusual 'pseudocatalytic' properties. Enzymes are known to catalyze more than 5,000 biochemical reaction types. Other biocatalysts are catalytic RNA molecules, called ribozymes. Enzymes' specificity comes from their unique three-dimensional structures. Like all catalysts, enzymes increase the reaction ra ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phosphate
In chemistry, a phosphate is an anion, salt, functional group or ester derived from a phosphoric acid. It most commonly means orthophosphate, a derivative of orthophosphoric acid . The phosphate or orthophosphate ion is derived from phosphoric acid by the removal of three protons . Removal of one or two protons gives the dihydrogen phosphate ion and the hydrogen phosphate ion ion, respectively. These names are also used for salts of those anions, such as ammonium dihydrogen phosphate and trisodium phosphate. File:3-phosphoric-acid-3D-balls.png, Phosphoricacid File:2-dihydrogenphosphate-3D-balls.png, Dihydrogenphosphate File:1-hydrogenphosphate-3D-balls.png, Hydrogenphosphate File:0-phosphate-3D-balls.png, Phosphate In organic chemistry, phosphate or orthophosphate is an organophosphate, an ester of orthophosphoric acid of the form where one or more hydrogen atoms are replaced by organic groups. An example is trimethyl phosphate, . The term also refers to the triv ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Methionine Salvage Pathway And Polyamine Pathways
Methionine (symbol Met or M) () is an essential amino acid in humans. As the precursor of other amino acids such as cysteine and taurine, versatile compounds such as SAM-e, and the important antioxidant glutathione, methionine plays a critical role in the metabolism and health of many species, including humans. It is encoded by the codon AUG. Methionine is also an important part of angiogenesis, the growth of new blood vessels. Supplementation may benefit those suffering from copper poisoning. Overconsumption of methionine, the methyl group donor in DNA methylation, is related to cancer growth in a number of studies. Methionine was first isolated in 1921 by John Howard Mueller. Biochemical details Methionine (abbreviated as Met or M; encoded by the codon AUG) is an α-amino acid that is used in the biosynthesis of proteins. It contains a carboxyl group (which is in the deprotonated −COO− form under biological pH conditions), an amino group (which is in the protonated fo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Binding Site
In biochemistry and molecular biology, a binding site is a region on a macromolecule such as a protein that binds to another molecule with specificity. The binding partner of the macromolecule is often referred to as a ligand. Ligands may include other proteins (resulting in a protein-protein interaction), enzyme substrates, second messengers, hormones, or allosteric modulators. The binding event is often, but not always, accompanied by a conformational change that alters the protein's function. Binding to protein binding sites is most often reversible (transient and non-covalent), but can also be covalent reversible or irreversible. Function Binding of a ligand to a binding site on protein often triggers a change in conformation in the protein and results in altered cellular function. Hence binding site on protein are critical parts of signal transduction pathways. Types of ligands include neurotransmitters, toxins, neuropeptides, and steroid hormones. Binding sites in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
C-terminus
The C-terminus (also known as the carboxyl-terminus, carboxy-terminus, C-terminal tail, C-terminal end, or COOH-terminus) is the end of an amino acid chain (protein or polypeptide), terminated by a free carboxyl group (-COOH). When the protein is translated from messenger RNA, it is created from N-terminus to C-terminus. The convention for writing peptide sequences is to put the C-terminal end on the right and write the sequence from N- to C-terminus. Chemistry Each amino acid has a carboxyl group and an amine group. Amino acids link to one another to form a chain by a dehydration reaction which joins the amine group of one amino acid to the carboxyl group of the next. Thus polypeptide chains have an end with an unbound carboxyl group, the C-terminus, and an end with an unbound amine group, the N-terminus. Proteins are naturally synthesized starting from the N-terminus and ending at the C-terminus. Function C-terminal retention signals While the N-terminus of a protein often c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Active Site
In biology and biochemistry, the active site is the region of an enzyme where substrate molecules bind and undergo a chemical reaction. The active site consists of amino acid residues that form temporary bonds with the substrate (binding site) and residues that catalyse a reaction of that substrate (catalytic site). Although the active site occupies only ~10–20% of the volume of an enzyme, it is the most important part as it directly catalyzes the chemical reaction. It usually consists of three to four amino acids, while other amino acids within the protein are required to maintain the tertiary structure of the enzymes. Each active site is evolved to be optimised to bind a particular substrate and catalyse a particular reaction, resulting in high specificity. This specificity is determined by the arrangement of amino acids within the active site and the structure of the substrates. Sometimes enzymes also need to bind with some cofactors to fulfil their function. The active si ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alpha Helix
The alpha helix (α-helix) is a common motif in the secondary structure of proteins and is a right hand-helix conformation in which every backbone N−H group hydrogen bonds to the backbone C=O group of the amino acid located four residues earlier along the protein sequence. The alpha helix is also called a classic Pauling–Corey–Branson α-helix. The name 3.613-helix is also used for this type of helix, denoting the average number of residues per helical turn, with 13 atoms being involved in the ring formed by the hydrogen bond. Among types of local structure in proteins, the α-helix is the most extreme and the most predictable from sequence, as well as the most prevalent. Discovery In the early 1930s, William Astbury showed that there were drastic changes in the X-ray fiber diffraction of moist wool or hair fibers upon significant stretching. The data suggested that the unstretched fibers had a coiled molecular structure with a characteristic repeat of ≈. Astb ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Beta Sheet
The beta sheet, (β-sheet) (also β-pleated sheet) is a common motif of the regular protein secondary structure. Beta sheets consist of beta strands (β-strands) connected laterally by at least two or three backbone hydrogen bonds, forming a generally twisted, pleated sheet. A β-strand is a stretch of polypeptide chain typically 3 to 10 amino acids long with backbone in an extended conformation. The supramolecular association of β-sheets has been implicated in the formation of the fibrils and protein aggregates observed in amyloidosis, notably Alzheimer's disease. History The first β-sheet structure was proposed by William Astbury in the 1930s. He proposed the idea of hydrogen bonding between the peptide bonds of parallel or antiparallel extended β-strands. However, Astbury did not have the necessary data on the bond geometry of the amino acids in order to build accurate models, especially since he did not then know that the peptide bond was planar. A refined versi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |