|
METAP2
Methionine aminopeptidase 2 is an enzyme that in humans is encoded by the ''METAP2'' gene. Methionine aminopeptidase 2, a member of the dimetallohydrolase family, is a cytosolic metalloenzyme that catalyzes the hydrolytic removal of N-terminal methionine residues from nascent proteins. * peptide-methionine \rightleftharpoons peptide + methionine MetAP2 is found in all organisms and is especially important because of its critical role in tissue repair and protein degradation. Furthermore, MetAP2 is of particular interest because the enzyme plays a key role in angiogenesis, the growth of new blood vessels, which is necessary for the progression of diseases including solid tumor cancers and rheumatoid arthritis. MetAP2 is also the target of two groups of anti-angiogenic natural products, ovalicin and fumagillin, and their analogs such as beloranib. Structure In living organisms, the start codon that initiates protein synthesis codes for either methionine (eukaryotes) or f ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Beloranib
Beloranib is a former drug candidate for the treatment of obesity. It was discovered by CKD Pharmaceuticals and its clinical development was led by Zafgen. Drug development was halted in 2016 after deaths during clinical trials. Mechanism of action Beloranib, an analog of the natural chemical compound fumagillin, is an inhibitor of the enzyme METAP2 Methionine aminopeptidase 2 is an enzyme that in humans is encoded by the ''METAP2'' gene. Methionine aminopeptidase 2, a member of the dimetallohydrolase family, is a cytosolic metalloenzyme that catalyzes the hydrolytic removal of N-terminal m .... It was originally designed as angiogenesis inhibitor for the treatment of cancer. However, once the potential anti-obesity effects of METAP2 inhibition became apparent, the clinical development began to focus on these effects and beloranib has shown positive results in preliminary clinical trials for this indication. Clinical trials A Phase I trial was published in 2013, finding ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fumagillin
Fumagillin is a complex biomolecule and used as an antimicrobial agent. It was isolated in 1949 from the microbial organism ''Aspergillus fumigatus''. Uses In animals It was originally used against microsporidian parasites ''Nosema apis'' infections in honey bees. Some studies found it to be effective against some myxozoan parasites, including ''Myxobolus cerebralis,'' an important parasite of fish; however, in the more rigorous tests required for U.S. Food and Drug Administration approval, it was ineffective. There are reports that fumagillin controls ''Nosema ceranae'', which has recently been hypothesized as a possible cause of colony collapse disorder. The latest report, however, has shown it to be ineffective against ''N. ceranae''. Fumagillin is also investigated as an inhibitor of malaria parasite growth. In humans Fumagillin has been used in the treatment of microsporidiosis. It is also an amebicide. Fumagillin can block blood vessel formation by binding to an enzyme m ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Enzyme
Enzymes () are proteins that act as biological catalysts by accelerating chemical reactions. The molecules upon which enzymes may act are called substrate (chemistry), substrates, and the enzyme converts the substrates into different molecules known as product (chemistry), products. Almost all metabolism, metabolic processes in the cell (biology), cell need enzyme catalysis in order to occur at rates fast enough to sustain life. Metabolic pathways depend upon enzymes to catalyze individual steps. The study of enzymes is called ''enzymology'' and the field of pseudoenzyme, pseudoenzyme analysis recognizes that during evolution, some enzymes have lost the ability to carry out biological catalysis, which is often reflected in their amino acid sequences and unusual 'pseudocatalytic' properties. Enzymes are known to catalyze more than 5,000 biochemical reaction types. Other biocatalysts are Ribozyme, catalytic RNA molecules, called ribozymes. Enzymes' Chemical specificity, specific ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Monomeric
In chemistry, a monomer ( ; ''wikt:mono-, mono-'', "one" + ''wikt:-mer, -mer'', "part") is a molecule that can chemical reaction, react together with other monomer molecules to form a larger polymer chain or three-dimensional network in a process called polymerization. Classification Monomers can be classified in many ways. They can be subdivided into two broad classes, depending on the kind of the polymer that they form. Monomers that participate in condensation polymerization have a different stoichiometry than monomers that participate in addition polymerization: : Other classifications include: *natural vs synthetic monomers, e.g. glycine vs caprolactam, respectively *polar vs nonpolar monomers, e.g. vinyl acetate vs ethylene, respectively *cyclic vs linear, e.g. ethylene oxide vs ethylene glycol, respectively The polymerization of one kind of monomer gives a homopolymer. Many polymers are copolymers, meaning that they are derived from two different monomers. In the cas ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phosphatases
In biochemistry, a phosphatase is an enzyme that uses water to cleave a phosphoric acid monoester into a phosphate ion and an alcohol. Because a phosphatase enzyme catalyzes the hydrolysis of its substrate, it is a subcategory of hydrolases. Phosphatase enzymes are essential to many biological functions, because phosphorylation (e.g. by protein kinases) and dephosphorylation (by phosphatases) serve diverse roles in cellular regulation and signaling. Whereas phosphatases remove phosphate groups from molecules, kinases catalyze the transfer of phosphate groups to molecules from ATP. Together, kinases and phosphatases direct a form of post-translational modification that is essential to the cell's regulatory network. Phosphatase enzymes are not to be confused with phosphorylase enzymes, which catalyze the transfer of a phosphate group from hydrogen phosphate to an acceptor. Due to their prevalence in cellular regulation, phosphatases are an area of interest for pharmaceutical res ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Arginase
Arginase (, ''arginine amidinase'', ''canavanase'', ''L-arginase'', ''arginine transamidinase'') is a manganese-containing enzyme. The reaction catalyzed by this enzyme is: : arginine + H2O → ornithine + urea It is the final enzyme of the urea cycle. It is ubiquitous to all domains of life. Structure and function Arginase belong to the ureohydrolase family of enzymes. Arginase catalyzes the fifth and final step in the urea cycle, a series of biochemical reactions in mammals during which the body disposes of harmful ammonia. Specifically, arginase converts L-arginine into L-ornithine and urea. Mammalian arginase is active as a trimer, but some bacterial arginases are hexameric. The enzyme requires a two-molecule metal cluster of manganese in order to maintain proper function. These Mn2+ ions coordinate with water, orienting and stabilizing the molecule and allowing water to act as a nucleophile and attack L-arginine, hydrolyzing it into ornithine and urea. In most mammals, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Urease
Ureases (), functionally, belong to the superfamily of amidohydrolases and phosphotriesterases. Ureases are found in numerous bacteria, fungi, algae, plants, and some invertebrates, as well as in soils, as a soil enzyme. They are nickel-containing metalloenzymes of high molecular weight. These enzymes catalyze the hydrolysis of urea into carbon dioxide and ammonia: : (NH2)2CO + H2O CO2 + 2NH3 The hydrolysis of urea occurs in two stages. In the first stage, ammonia and carbamic acid are produced. The carbamate spontaneously and rapidly hydrolyzes to ammonia and carbonic acid. Urease activity increases the pH of its environment as ammonia is produced, which is basic. History Its activity was first identified in 1876 by Frédéric Alphonse Musculus as a soluble ferment. In 1926, James B. Sumner, showed that urease is a protein by examining its crystallized form. Sumner's work was the first demonstration that a protein can function as an enzyme and led eventually to the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
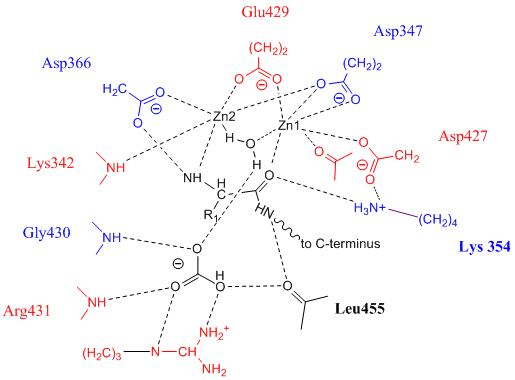
Leucine Aminopeptidase
Leucyl aminopeptidases (, ''leucine aminopeptidase'', ''LAPs'', ''leucyl peptidase'', ''peptidase S'', ''cytosol aminopeptidase'', ''cathepsin III'', ''L-leucine aminopeptidase'', ''leucinaminopeptidase'', ''leucinamide aminopeptidase'', ''FTBL proteins'', ''proteinates FTBL'', ''aminopeptidase II'', ''aminopeptidase III'', ''aminopeptidase I'') are enzymes that preferentially catalyze the hydrolysis of leucine residues at the N-terminus of peptides and proteins. Other N-terminal residues can also be cleaved, however. LAPs have been found across superkingdoms. Identified LAPs include human LAP, bovine lens LAP, porcine LAP, ''Escherichia coli'' (''E. coli'') LAP (also known as PepA or XerB), and the solanaceous-specific acidic LAP (LAP-A) in tomato (''Solanum lycopersicum''). Enzyme description, structure, and active site The active sites in PepA and in bovine lens LAP have been found to be similar. Shown in the picture below is the proposed model for the active site of LAP-A i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ribonucleotide Reductase
Ribonucleotide reductase (RNR), also known as ribonucleoside diphosphate reductase (rNDP), is an enzyme that catalyzes the formation of deoxyribonucleotides from ribonucleotides. It catalyzes this formation by removing the 2'-hydroxyl group of the ribose ring of nucleoside diphosphates. This reduction produces deoxyribonucleotides. Deoxyribonucleotides in turn are used in the synthesis of DNA. The reaction catalyzed by RNR is strictly conserved in all living organisms. Furthermore, RNR plays a critical role in regulating the total rate of DNA synthesis so that DNA to cell mass is maintained at a constant ratio during cell division and DNA repair. A somewhat unusual feature of the RNR enzyme is that it catalyzes a reaction that proceeds via a free radical mechanism of action. The substrates for RNR are ADP, GDP, CDP and UDP. dTDP (deoxythymidine diphosphate) is synthesized by another enzyme (thymidylate kinase) from dTMP (deoxythymidine monophosphate). Structure Ribonucleoti ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hemerythrin
Hemerythrin (also spelled haemerythrin; grc, αἷμα, haîma, blood, grc, ἐρυθρός, erythrós, red) is an oligomeric protein responsible for oxygen (O2) transport in the marine invertebrate phyla of sipunculids, priapulids, brachiopods, and in a single annelid worm genus, ''Magelona''. Myohemerythrin is a monomeric O2-binding protein found in the muscles of marine invertebrates. Hemerythrin and myohemerythrin are essentially colorless when deoxygenated, but turn a violet-pink in the oxygenated state. Hemerythrin does not, as the name might suggest, contain a heme. The names of the blood oxygen transporters hemoglobin, hemocyanin, hemerythrin, do not refer to the heme group (only found in globins), instead these names are derived from the Greek word for blood. Hemerythrin may also contribute to innate immunity and anterior tissue regeneration in certain worms. O2 binding mechanism The mechanism of dioxygen binding is unusual. Most O2 carriers operate via formation of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |