|
MAC, The Mitochondrial Apoptosis-Induced Channel
The mitochondrial apoptosis-induced channel (or MAC), is an early marker of the onset of apoptosis. This ion channel is formed on the outer mitochondrial membrane in response to certain apoptotic stimuli. MAC activity is detected by patch clamping mitochondria from apoptotic cells at the time of cytochrome c release. Members of the Bcl-2 protein family regulate apoptosis by controlling the formation of MAC: the pro-apoptotic members Bax and/or Bak form MAC, whereas the anti-apoptotic members like Bcl-2 or Bcl-xL B-cell lymphoma-extra large (Bcl-xL), encoded by the BCL2-like 1 gene, is a transmembrane molecule in the mitochondria. It is a member of the Bcl-2 family of proteins, and acts as an anti-apoptotic protein by preventing the release of mitochondr ... prevent MAC formation. Once formed, MAC mediates the release of cytochrome c to the cytosol, triggering the commitment step of the mitochondrial apoptotic cascade. Depletion of MAC activity is accomplished pharmacolog ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Apoptosis
Apoptosis (from grc, ἀπόπτωσις, apóptōsis, 'falling off') is a form of programmed cell death that occurs in multicellular organisms. Biochemical events lead to characteristic cell changes (morphology) and death. These changes include blebbing, cell shrinkage, nuclear fragmentation, chromatin condensation, DNA fragmentation, and mRNA decay. The average adult human loses between 50 and 70 billion cells each day due to apoptosis. For an average human child between eight and fourteen years old, approximately twenty to thirty billion cells die per day. In contrast to necrosis, which is a form of traumatic cell death that results from acute cellular injury, apoptosis is a highly regulated and controlled process that confers advantages during an organism's life cycle. For example, the separation of fingers and toes in a developing human embryo occurs because cells between the digits undergo apoptosis. Unlike necrosis, apoptosis produces cell fragments called apoptotic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ion Channel
Ion channels are pore-forming membrane proteins that allow ions to pass through the channel pore. Their functions include establishing a resting membrane potential, shaping action potentials and other electrical signals by gating the flow of ions across the cell membrane, controlling the flow of ions across secretory and epithelial cells, and regulating cell volume. Ion channels are present in the membranes of all cells. Ion channels are one of the two classes of ionophoric proteins, the other being ion transporters. The study of ion channels often involves biophysics, electrophysiology, and pharmacology, while using techniques including voltage clamp, patch clamp, immunohistochemistry, X-ray crystallography, fluoroscopy, and RT-PCR. Their classification as molecules is referred to as channelomics. Basic features There are two distinctive features of ion channels that differentiate them from other types of ion transporter proteins: #The rate of ion transport through the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Outer Mitochondrial Membrane
A mitochondrion (; ) is an organelle found in the cells of most Eukaryotes, such as animals, plants and fungi. Mitochondria have a double membrane structure and use aerobic respiration to generate adenosine triphosphate (ATP), which is used throughout the cell as a source of chemical energy. They were discovered by Albert von Kölliker in 1857 in the voluntary muscles of insects. The term ''mitochondrion'' was coined by Carl Benda in 1898. The mitochondrion is popularly nicknamed the "powerhouse of the cell", a phrase coined by Philip Siekevitz in a 1957 article of the same name. Some cells in some multicellular organisms lack mitochondria (for example, mature mammalian red blood cells). A large number of unicellular organisms, such as microsporidia, parabasalids and diplomonads, have reduced or transformed their mitochondria into other structures. One eukaryote, ''Monocercomonoides'', is known to have completely lost its mitochondria, and one multicellular organism, '' Henn ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Patch Clamp
The patch clamp technique is a laboratory technique in electrophysiology used to study ionic currents in individual isolated living cells, tissue sections, or patches of cell membrane. The technique is especially useful in the study of excitable cells such as neurons, cardiomyocytes, muscle fibers, and pancreatic beta cells, and can also be applied to the study of bacterial ion channels in specially prepared giant spheroplasts. Patch clamping can be performed using the voltage clamp technique. In this case, the voltage across the cell membrane is controlled by the experimenter and the resulting currents are recorded. Alternatively, the current clamp technique can be used. In this case, the current passing across the membrane is controlled by the experimenter and the resulting changes in voltage are recorded, generally in the form of action potentials. Erwin Neher and Bert Sakmann developed the patch clamp in the late 1970s and early 1980s. This discovery made it possible to ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cytochrome C
The cytochrome complex, or cyt ''c'', is a small hemeprotein found loosely associated with the inner membrane of the mitochondrion. It belongs to the cytochrome c family of proteins and plays a major role in cell apoptosis. Cytochrome c is highly water-soluble, unlike other cytochromes, and is an essential component of the respiratory electron transport chain, where it carries one electron. It is capable of undergoing oxidation and reduction as its iron atom converts between the ferrous and ferric forms, but does not bind oxygen. It transfers electrons between Complexes III (Coenzyme Q – Cyt c reductase) and IV (Cyt c oxidase). In humans, cytochrome c is encoded by the ''CYCS'' gene. Species distribution Cytochrome c is a highly conserved protein across the spectrum of eukaryotic species, found in plants, animals, fungi, and many unicellular organisms. This, along with its small size (molecular weight about 12,000 daltons), makes it useful in studies of cladistics. Cyt ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bcl-2
Bcl-2 (B-cell lymphoma 2), encoded in humans by the ''BCL2'' gene, is the founding member of the Bcl-2 family of regulator proteins that regulate cell death (apoptosis), by either inhibiting (anti-apoptotic) or inducing (pro-apoptotic) apoptosis. It was the first apoptosis regulator identified in any organism. Bcl-2 derives its name from ''B-cell lymphoma 2'', as it is the second member of a range of proteins initially described in chromosomal translocations involving chromosomes 14 and 18 in follicular lymphomas. Orthologs (such as ''Bcl2'' in mice) have been identified in numerous mammals for which complete genome data are available. Like BCL3, BCL5, BCL6, BCL7A, BCL9, and BCL10, it has clinical significance in lymphoma. Isoforms The two isoforms of Bcl-2, Isoform 1, and Isoform 2, exhibit a similar fold. However, results in the ability of these isoforms to bind to the BAD and BAK proteins, as well as in the structural topology and electrostatic potential of the binding ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bcl-2-associated X Protein
Apoptosis regulator BAX, also known as bcl-2-like protein 4, is a protein that in humans is encoded by the ''BAX'' gene. ''BAX'' is a member of the Bcl-2 gene family. BCL2 family members form hetero- or homodimers and act as anti- or pro-apoptotic regulators that are involved in a wide variety of cellular activities. This protein forms a heterodimer with BCL2, and functions as an apoptotic activator. This protein is reported to interact with, and increase the opening of, the mitochondrial voltage-dependent anion channel (VDAC), which leads to the loss in membrane potential and the release of cytochrome c. The expression of this gene is regulated by the tumor suppressor P53 and has been shown to be involved in P53-mediated apoptosis. Structure The ''BAX'' gene was the first identified pro-apoptotic member of the Bcl-2 protein family. Bcl-2 family members share one or more of the four characteristic domains of homology entitled the Bcl-2 homology (BH) domains (named BH1, BH2, B ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bcl-2 Homologous Antagonist Killer
Bcl-2 homologous antagonist/killer is a protein that in humans is encoded by the ''BAK1'' gene on chromosome 6. The protein encoded by this gene belongs to the BCL2 protein family. BCL2 family members form oligomers or heterodimers and act as anti- or pro-apoptotic regulators that are involved in a wide variety of cellular activities. This protein localizes to mitochondria, and functions to induce apoptosis. It interacts with and accelerates the opening of the mitochondrial voltage-dependent anion channel, which leads to a loss in membrane potential and the release of cytochrome c. This protein also interacts with the tumor suppressor P53 after exposure to cell stress. Structure BAK1 is a pro-apoptotic Bcl-2 protein containing four Bcl-2 homology (BH) domains: BH1, BH2, BH3, and BH4. These domains are composed of nine α-helices, with a hydrophobic α-helix core surrounded by amphipathic helices and a transmembrane C-terminal α-helix anchored to the mitochondrial outer membran ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bcl-xL
B-cell lymphoma-extra large (Bcl-xL), encoded by the BCL2-like 1 gene, is a transmembrane molecule in the mitochondria. It is a member of the Bcl-2 family of proteins, and acts as an anti-apoptotic protein by preventing the release of mitochondrial contents such as cytochrome c, which leads to caspase activation and ultimately, programmed cell death. Function It is a well-established concept in the field of apoptosis that relative amounts of pro- and anti-survival Bcl-2 family of proteins determine whether the cell will undergo cell death; if more Bcl-xL is present, then pores are non-permeable to pro-apoptotic molecules and the cell survives. However, if Bax and Bak become activated, and Bcl-xL is sequestered away by gatekeeper BH3-only factors (e.g. Bim) causing a pore to form, cytochrome c is released leading to initiation of caspase cascade and apoptotic events. While the exact signaling pathway of Bcl-xL is still not known, it is believed that Bcl-xL differs highly from ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
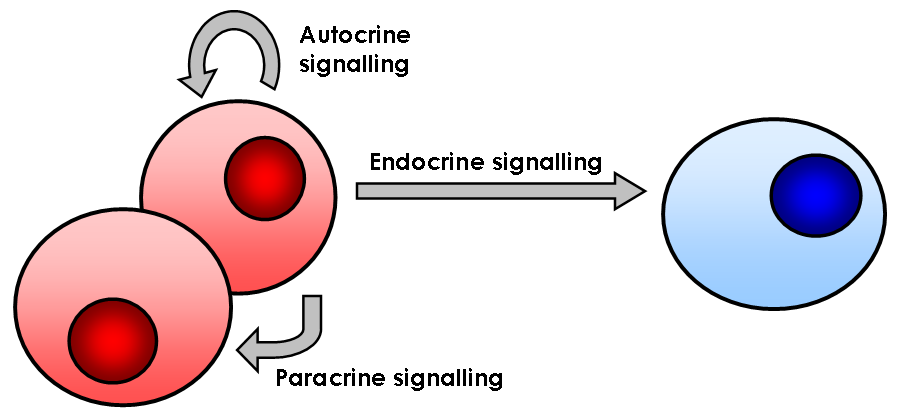
Cell Signaling
In biology, cell signaling (cell signalling in British English) or cell communication is the ability of a cell to receive, process, and transmit signals with its environment and with itself. Cell signaling is a fundamental property of all cellular life in prokaryotes and eukaryotes. Signals that originate from outside a cell (or extracellular signals) can be physical agents like mechanical pressure, voltage, temperature, light, or chemical signals (e.g., small molecules, peptides, or gas). Cell signaling can occur over short or long distances, and as a result can be classified as autocrine, juxtacrine, intracrine, paracrine, or endocrine. Signaling molecules can be synthesized from various biosynthetic pathways and released through passive or active transports, or even from cell damage. Receptors play a key role in cell signaling as they are able to detect chemical signals or physical stimuli. Receptors are generally proteins located on the cell surface or within the interio ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Programmed Cell Death
Programmed cell death (PCD; sometimes referred to as cellular suicide) is the death of a cell as a result of events inside of a cell, such as apoptosis or autophagy. PCD is carried out in a biological process, which usually confers advantage during an organism's lifecycle. For example, the differentiation of fingers and toes in a developing human embryo occurs because cells between the fingers apoptose; the result is that the digits are separate. PCD serves fundamental functions during both plant and animal tissue development. Apoptosis and autophagy are both forms of programmed cell death. Necrosis is the death of a cell caused by external factors such as trauma or infection and occurs in several different forms. Necrosis was long seen as a non-physiological process that occurs as a result of infection or injury, but in the 2000s, a form of programmed necrosis, called necroptosis, was recognized as an alternative form of programmed cell death. It is hypothesized that necroptosis ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |