|
Lysin
Lysins, also known as endolysins or murein hydrolases, are hydrolytic enzymes produced by bacteriophages in order to cleave the host's cell wall during the final stage of the lytic cycle. Lysins are highly evolved enzymes that are able to target one of the five bonds in peptidoglycan (murein), the main component of bacterial cell walls, which allows the release of progeny virions from the lysed cell. Cell-wall-containing Archaea are also lysed by specialized pseudomurein-cleaving lysins, while most archaeal viruses employ alternative mechanisms. Similarly, not all bacteriophages synthesize lysins: some small single-stranded DNA and RNA phages produce membrane proteins that activate the host's autolytic mechanisms such as autolysins. Lysins are being used as antibacterial agents due to their high effectiveness and specificity in comparison with antibiotics, which are susceptible to bacterial resistance. Structure Double-stranded DNA phage lysins tend to lie within the 25 to ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Autolysin
Autolysins are endogenous lytic enzymes that break down the peptidoglycan components of biological cells which enables the separation of daughter cells following cell division. They are involved in cell growth, cell wall metabolism, cell division and separation, as well as peptidoglycan turnover and have similar functions to lysozymes. Autolysin is formed from the precursor gene, Atl. Amidases (EC 3.5.1.28), gametolysin (EC 3.4.24.38), and glucosaminidase are considered as types of autolysins. Function and mechanisms Autolysins exist in all bacteria containing peptidoglycan and are potentially considered as lethal enzymes when uncontrolled. They target the glycosidic bonds as well as the cross-linked peptides of the peptidoglycan matrix. The peptidoglycan matrix functions for cell wall stability to protect from turgor changes and carries out function for immunological defense. These enzymes break down the peptidoglycan matrix in small sections to allow for peptidoglycan biosynth ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
OBPgp279
OBPgp279 (OBP genome protein 279) is an endolysin that hydrolyzes peptidoglycan, a major constituent in bacterial membrane. OBPgp279 is found in ''Pseudomonas fluorescens'' phage OBP, which belongs in the ''Myoviridae'' family of bacteriophages. Because of its role in hydrolyzing the peptidoglycan layer, OBPgp279 is a key enzyme in the lytic cycle of the OBP bacteriophage; it allows the bacteriophage to lyse its host internally to escape. Unlike other endolysins, OBPgp279 does not rely on holins to perforate the inner bacterial membrane in order to reach the peptidoglycan layer. Although OBPgp279 is not a well-studied enzyme, it has garnered interest as a potential antibacterial protein due to its activity against multidrug-resistant gram-negative bacteria. Predicted enzyme mechanism The mechanism of OBPgp279 is predicted to be part of glycoside hydrolase family 19 (GH19) due to the presence of a conserved sequence motif (general sequence motif = HYG-R-G- Pζ-Q- L T HYW ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lytic Cycle
The lytic cycle ( ) is one of the two cycles of viral reproduction (referring to bacterial viruses or bacteriophages), the other being the lysogenic cycle. The lytic cycle results in the destruction of the infected cell and its membrane. Bacteriophages that only use the lytic cycle are called virulent phages (in contrast to temperate phages). In the lytic cycle, the viral DNA exists as a separate free floating molecule within the bacterial cell, and replicates separately from the host bacterial DNA, whereas in the lysogenic cycle, the viral DNA is located within the host DNA. This is the key difference between the lytic and lysogenic (bacterio)phage cycles. However, in both cases the virus/phage replicates using the host DNA machinery. Description The lytic cycle, which is also commonly referred to as the "reproductive cycle" of the bacteriophage, is a six-stage cycle. The six stages are: attachment, penetration, transcription, biosynthesis, maturation, and lysis. # Attachment � ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
N-acetylmuramoyl-L-alanine Amidase
In enzymology, a N-acetylmuramoyl-L-alanine amidase () is an enzyme that catalyzes a chemical reaction that cleaves the link between N-acetylmuramoyl residues and L-amino acid residues in certain cell-wall glycopeptides. This enzyme belongs to the family of hydrolases, specifically those acting on carbon-nitrogen bonds other than peptide bonds in linear amides. The systematic name of this enzyme class is peptidoglycan amidohydrolase. Other names in common use include acetylmuramyl-L-alanine amidase, N-acetylmuramyl-L-alanine amidase, N-acylmuramyl-L-alanine amidase, acetylmuramoyl-alanine amidase, N-acetylmuramic acid L-alanine amidase, acetylmuramyl-alanine amidase, N-acetylmuramylalanine amidase, N-acetylmuramoyl-L-alanine amidase type I, and N-acetylmuramoyl-L-alanine amidase type II. This enzyme participates in peptidoglycan biosynthesis. Autolysins and some phage lysins are examples of N-acetylmuramoyl-L-alanine amidases. See also * Phage lysins * Autolysin Autoly ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Holin
Holins are a diverse group of small proteins produced by dsDNA bacteriophages in order to trigger and control the degradation of the host's cell wall at the end of the lytic cycle. Holins form pores in the host's cell membrane, allowing lysins to reach and degrade peptidoglycan, a component of bacterial cell walls. Holins have been shown to regulate the timing of lysis with great precision. Over 50 unrelated gene families encode holins, making them the most diverse group of proteins with common function. Together with lysins, holins are being studied for their potential use as antibacterial agents. While canonical holins act by forming large pores, pinholins such as the S protein of lambdoid phage 21 act by forming heptameric channels that depolarize the bacterial membrane. They are associated with SAR endolysins, which remain inactive in the periplasm prior to the depolarization of the membrane. Viruses that infect eukaryotic cells may use similar channel-forming proteins calle ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Peptidoglycan
Peptidoglycan or murein is a unique large macromolecule, a polysaccharide, consisting of sugars and amino acids that forms a mesh-like peptidoglycan layer outside the plasma membrane, the rigid cell wall (murein sacculus) characteristic of most bacteria (domain ''Bacteria''). The sugar component consists of alternating residues of β-(1,4) linked ''N''-acetylglucosamine (NAG) and ''N''-acetylmuramic acid (NAM). Attached to the ''N''-acetylmuramic acid is a oligopeptide chain made of three to five amino acids. The peptide chain can be cross-linked to the peptide chain of another strand forming the 3D mesh-like layer. Peptidoglycan serves a structural role in the bacterial cell wall, giving structural strength, as well as counteracting the osmotic pressure of the cytoplasm. This repetitive linking results in a dense peptidoglycan layer which is critical for maintaining cell form and withstanding high osmotic pressures, and it is regularly replaced by peptidoglycan production. Peptid ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bacteriophage
A bacteriophage (), also known informally as a ''phage'' (), is a duplodnaviria virus that infects and replicates within bacteria and archaea. The term was derived from "bacteria" and the Greek φαγεῖν ('), meaning "to devour". Bacteriophages are composed of proteins that encapsulate a DNA or RNA genome, and may have structures that are either simple or elaborate. Their genomes may encode as few as four genes (e.g. MS2) and as many as hundreds of genes. Phages replicate within the bacterium following the injection of their genome into its cytoplasm. Bacteriophages are among the most common and diverse entities in the biosphere. Bacteriophages are ubiquitous viruses, found wherever bacteria exist. It is estimated there are more than 1031 bacteriophages on the planet, more than every other organism on Earth, including bacteria, combined. Viruses are the most abundant biological entity in the water column of the world's oceans, and the second largest component o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protein Domain
In molecular biology, a protein domain is a region of a protein's polypeptide chain that is self-stabilizing and that folds independently from the rest. Each domain forms a compact folded three-dimensional structure. Many proteins consist of several domains, and a domain may appear in a variety of different proteins. Molecular evolution uses domains as building blocks and these may be recombined in different arrangements to create proteins with different functions. In general, domains vary in length from between about 50 amino acids up to 250 amino acids in length. The shortest domains, such as zinc fingers, are stabilized by metal ions or disulfide bridges. Domains often form functional units, such as the calcium-binding EF hand domain of calmodulin. Because they are independently stable, domains can be "swapped" by genetic engineering between one protein and another to make chimeric proteins. Background The concept of the domain was first proposed in 1973 by Wetlaufer af ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lysozyme
Lysozyme (EC 3.2.1.17, muramidase, ''N''-acetylmuramide glycanhydrolase; systematic name peptidoglycan ''N''-acetylmuramoylhydrolase) is an antimicrobial enzyme produced by animals that forms part of the innate immune system. It is a glycoside hydrolase that catalyzes the following process: : Hydrolysis of (1→4)-β-linkages between ''N''-acetylmuramic acid and ''N''-acetyl-D-glucosamine residues in a peptidoglycan and between ''N''-acetyl-D-glucosamine residues in chitodextrins Peptidoglycan is the major component of gram-positive bacterial cell wall. This hydrolysis in turn compromises the integrity of bacterial cell walls causing lysis of the bacteria. Lysozyme is abundant in secretions including tears, saliva, human milk, and mucus. It is also present in cytoplasmic granules of the macrophages and the polymorphonuclear neutrophils (PMNs). Large amounts of lysozyme can be found in egg white. C-type lysozymes are closely related to α-lactalbumin in sequence and struc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Endopeptidase
Endopeptidase or endoproteinase are proteolytic peptidases that break peptide bonds of nonterminal amino acids (i.e. within the molecule), in contrast to exopeptidases, which break peptide bonds from end-pieces of terminal amino acids. For this reason, endopeptidases cannot break down peptides into monomers, while exopeptidases can break down proteins into monomers. A particular case of endopeptidase is the oligopeptidase, whose substrates are oligopeptides instead of proteins. They are usually very specific for certain amino acids. Examples of endopeptidases include: * Trypsin - cuts after Arg or Lys, unless followed by Pro. Very strict. Works best at pH 8. * Chymotrypsin - cuts after Phe, Trp, or Tyr, unless followed by Pro. Cuts more slowly after His, Met or Leu. Works best at pH 8. * Elastase - cuts after Ala, Gly, Ser, or Val, unless followed by Pro. * Thermolysin - cuts ''before'' Ile, Met, Phe, Trp, Tyr, or Val, unless ''preceded'' by Pro. Sometimes cuts after Ala, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
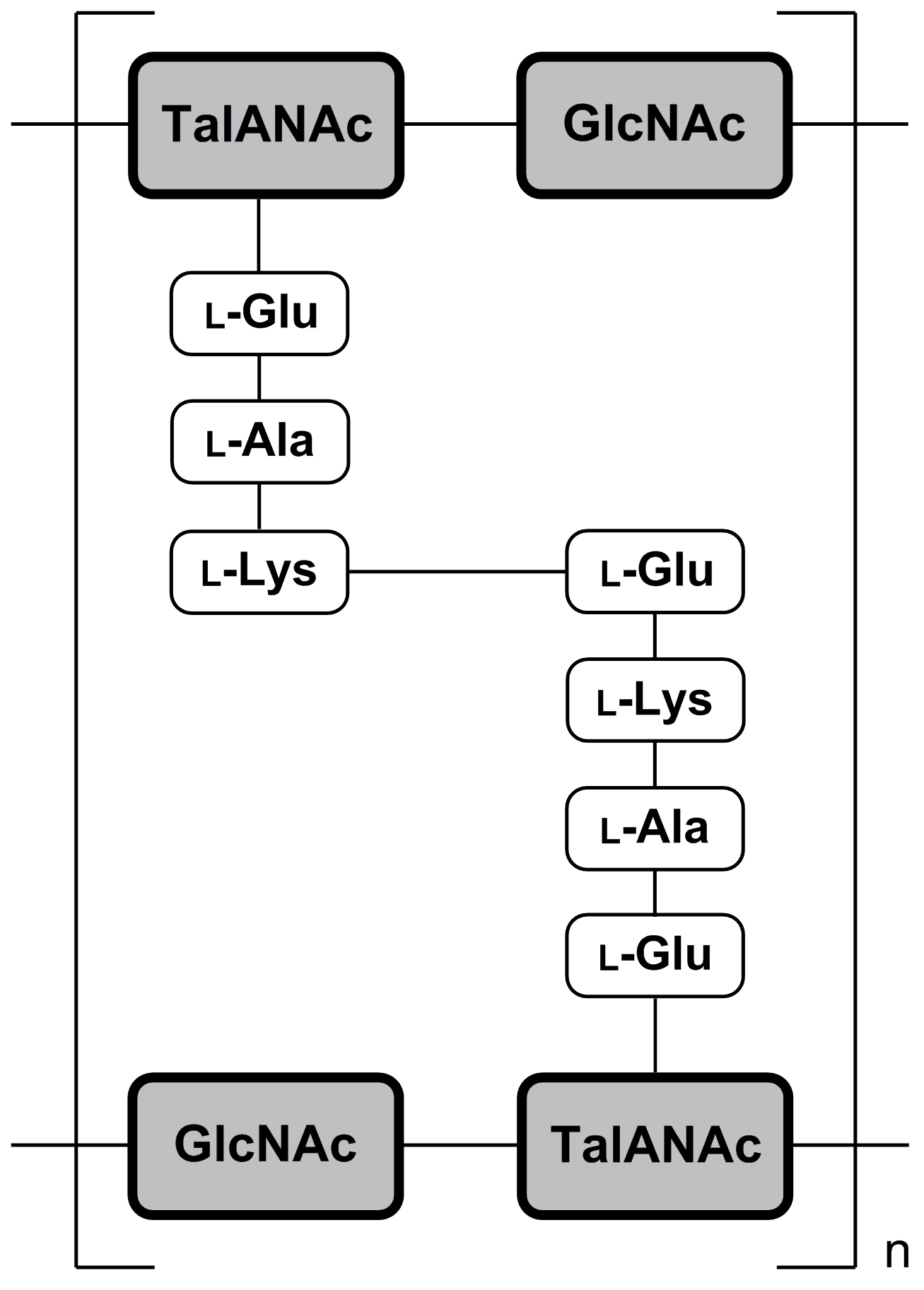
Pseudomurein
Pseudopeptidoglycan (also known as pseudomurein;White, David. (1995) ''The Physiology and Biochemistry of Prokaryotes'', pages 6, 12-21. (Oxford: Oxford University Press). . PPG hereafter) is a major cell wall component of some Archaea that differs from bacterial peptidoglycan in chemical structure, but resembles bacterial peptidoglycan in function and physical structure. Pseudopeptidoglycan, in general, is only present in a few methanogenic archaea. The basic components are N-acetylglucosamine and N-acetyltalosaminuronic acid (bacterial peptidoglycan containing ''N''-acetylmuramic acid instead), which are linked by β-1,3-glycosidic bonds. Lysozyme, a host defense mechanism present in human secretions (e.g. saliva and tears) breaks β-1,4-glycosidic bonds to degrade peptidoglycan. However, because pseudopeptidoglycan has β-1,3-glycosidic bonds, lysozyme is ineffective. It was thought from these large differences in cell wall chemistry that archaeal cell walls and bacterial cell ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Antibiotics
An antibiotic is a type of antimicrobial substance active against bacteria. It is the most important type of antibacterial agent for fighting bacterial infections, and antibiotic medications are widely used in the treatment and prevention of such infections. They may either kill or inhibit the growth of bacteria. A limited number of antibiotics also possess antiprotozoal activity. Antibiotics are not effective against viruses such as the common cold or influenza; drugs which inhibit viruses are termed antiviral drugs or antivirals rather than antibiotics. Sometimes, the term ''antibiotic''—literally "opposing life", from the Greek roots ἀντι ''anti'', "against" and βίος ''bios'', "life"—is broadly used to refer to any substance used against microbes, but in the usual medical usage, antibiotics (such as penicillin) are those produced naturally (by one microorganism fighting another), whereas non-antibiotic antibacterials (such as sulfonamides and an ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |