|
Lupeol
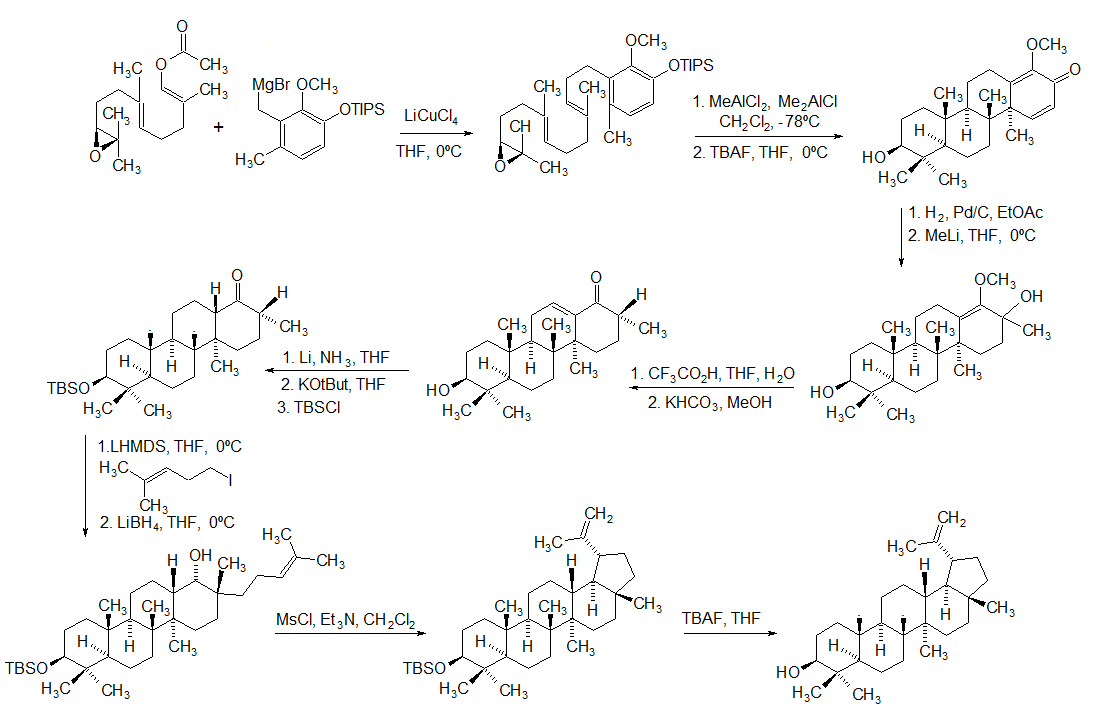
Lupeol is a pharmacologically active pentacyclic triterpenoid. It has several potential medicinal properties, like anticancer and anti-inflammatory activity. Natural occurrences Lupeol is found in a variety of plants, including mango, ''Acacia visco'' and ''Abronia villosa''. It is also found in dandelion coffee. Lupeol is present as a major component in ''Camellia japonica'' leaf. Total synthesis The first total synthesis of lupeol was reported by Gilbert Stork ''et al''. In 2009, Surendra and Corey reported a more efficient and enantioselective total synthesis of lupeol, starting from (''1E,5E'')-8- ''2S'')-3,3-dimethyloxiran-2-yl2,6-dimethylocta-1,5-dienyl acetate by use of a polycyclization. Biosynthesis Lupeol is produced by several organisms from squalene epoxide. Dammarane and baccharane skeletons are formed as intermediates. The reactions are catalyzed by the enzyme lupeol synthase. A recent study on the metabolomics of ''Camellia japonica'' leaf revealed ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
LUPEOL SYNTHESIS
Lupeol is a pharmacologically active pentacyclic triterpenoid. It has several potential medicinal properties, like anticancer and anti-inflammatory activity. Natural occurrences Lupeol is found in a variety of plants, including mango, ''Acacia visco'' and ''Abronia villosa''. It is also found in dandelion coffee. Lupeol is present as a major component in ''Camellia japonica'' leaf. Total synthesis The first total synthesis of lupeol was reported by Gilbert Stork ''et al''. In 2009, Surendra and Corey reported a more efficient and enantioselective total synthesis of lupeol, starting from (''1E,5E'')-8- ''2S'')-3,3-dimethyloxiran-2-yl2,6-dimethylocta-1,5-dienyl acetate by use of a polycyclization. Biosynthesis Lupeol is produced by several organisms from squalene epoxide. Dammarane and baccharane skeletons are formed as intermediates. The reactions are catalyzed by the enzyme lupeol synthase. A recent study on the metabolomics of ''Camellia japonica'' leaf revealed th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lupeol Synthase
Lupeol synthase (, ''LUPI'', ''BPW'', ''RcLUS'') is an enzyme with systematic name ''(3S)-2,3-epoxy-2,3-dihydrosqualene mutase (cyclizing, lupeol-forming)''. This enzyme catalyses the following chemical reaction : (3S)-2,3-epoxy-2,3-dihydrosqualene \rightleftharpoons lupeol Also forms some beta-amyrin The amyrins are three closely related natural chemical compounds of the triterpene class. They are designated α-amyrin (ursane skeleton), β-amyrin (oleanane skeleton) and δ-amyrin. Each is a pentacyclic triterpenol with the chemical formula C .... References External links * {{Portal bar, Biology, border=no EC 5.4.99 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Betulinic Acid
Betulinic acid is a naturally occurring pentacyclic triterpenoid which has antiretroviral, antimalarial, and anti-inflammatory properties, as well as a more recently discovered potential as an anticancer agent, by inhibition of topoisomerase. It is found in the bark of several species of plants, principally the white birch (''Betula pubescens'') from which it gets its name, but also the ber tree (''Ziziphus mauritiana''), selfheal (''Prunella vulgaris''), the tropical carnivorous plants '' Triphyophyllum peltatum'' and ''Ancistrocladus heyneanus'', '' Diospyros leucomelas'', a member of the persimmon family, '' Tetracera boiviniana'', the jambul (''Syzygium formosanum''), flowering quince (''Pseudocydonia sinensis'', former ''Chaenomeles sinensis KOEHNE''), (''Chaenomeles sinensis KOEHNE'' is now named ''Pseudocydonia sinensis'') rosemary, and ''Pulsatilla chinensis''. Antitumor activity In 1995, betulinic acid was reported as a selective inhibitor of human melanoma. Then it w ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acacia Visco
''Parasenegalia visco'' is a perennial tree found at higher elevations in northern Argentina, Bolivia, Chile and Peru. It has also been introduced to Africa. Common names for it include arca, visco, viscote, viscote blanco and viscote negro. It grows 6–25m tall and it has fragrant yellow flowers in the Spring. In Bolivia is found at an altitude of 1500–3000m. It has light to dark reddish brown twigs and small white flowers. It is cultivated for use in cabinetmaking. Methanol extract of ''Senegalia visco'' has been shown to have short-term and long-term anti-inflammatory effects in lab rats. Among the class of compounds characterized from ''S. visco'' leaves, the triterpenoid lupeol, α-amyrin and β-amyrin may be mainly responsible for the pharmacological activities.Pedernera AM, Guardia T, Calderón CE, Rotelli AE, de la Rocha NE, Saad JR, Verrilli MA, Aseff SG, Pelzer LE. Anti-inflammatory effect of ''Acacia visco'' extracts in animal models. Inflammopharmacology. 2010 O ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Abronia Villosa
''Abronia villosa'' is a species of sand-verbena known by the common names desert sand-verbena and chaparral sand-verbena. It is in the four o'clock plant family (Nyctaginaceae). It is native to sandy areas in the deserts of the southwestern United States and northern Mexico, associated with creosote-bush and coastal-sage scrub habitats. Description ''Abronia villosa'' is a short, hairy annual wildflower which grows in creeping prostrate masses along the ground. It has oval-shaped dull green leaves and many peduncles bearing rounded inflorescences of bright magenta or purplish-pink flowers. It grows in the sand of the deserts and coastlines. It has a very sweet fragrance, and is also very sticky. They usually grow between February and May. Chemistry The rotenoids abronione and boeravinone C, and the terpenoid lupeol can be found in ''A. villosa''. References Further reading * External links ''Abronia villosa'' — CalPhotos photo gallery {{Taxonbar, from=Q28 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Camellia Japonica
''Camellia japonica'', known as common camellia, or Japanese camellia, is a species of flowering plant in the family Theaceae. There are thousands of cultivars of ''C. japonica'' in cultivation, with many colors and forms of flowers. In the U.S. it is sometimes called japonica. In the wild, it is found in mainland China (Shandong, east Zhejiang), Taiwan, southern Korea and southwestern Japan. It grows in forests, at altitudes of around . Camellias are famous throughout East Asia; they are known as ''tsaa4 faa1'' (, lit. "tea flower") in Cantonese, ''cháhuā'' () in Mandarin Chinese, ''tsubaki'' () in Japanese, ''dongbaek-kkot'' () in Korean, and as ''hoa trà'' or ''hoa chè'' in Vietnamese. The leaves of this species are rich in anti-inflammatory terpenoids such as lupeol and squalene. Description ''Camellia japonica'' is a flowering tree or shrub, usually tall, but occasionally up to tall. Some cultivated varieties achieve a size of 72 m2 or more. The youngest ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mango
A mango is an edible stone fruit produced by the tropical tree '' Mangifera indica''. It is believed to have originated in the region between northwestern Myanmar, Bangladesh, and northeastern India. ''M. indica'' has been cultivated in South and Southeast Asia since ancient times resulting in two types of modern mango cultivars: the "Indian type" and the "Southeast Asian type". Other species in the genus ''Mangifera'' also produce edible fruits that are also called "mangoes", the majority of which are found in the Malesian ecoregion. Worldwide, there are several hundred cultivars of mango. Depending on the cultivar, mango fruit varies in size, shape, sweetness, skin color, and flesh color which may be pale yellow, gold, green, or orange. Mango is the national fruit of India, Pakistan and the Philippines, while the mango tree is the national tree of Bangladesh. Etymology The English word ''mango'' (plural "mangoes" or "mangos") originated in the 16th century from the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Triterpenoid
Triterpenes are a class of chemical compounds composed of three terpene units with the molecular formula C30H48; they may also be thought of as consisting of six isoprene units. Animals, plants and fungi all produce triterpenes, including squalene, the precursor to all steroids. Structures Triterpenes exist in a great variety of structures. Nearly 200 different skeletons have been identified. These skeletons may be broadly divided according to the number of rings present. In general pentacyclic structures (5 rings) tend to dominate. Squalene is biosynthesized through the head-to-head condensation of two farnesyl pyrophosphate units. This coupling converts a pair of C15 components into a C30 product. Squalene serves as precursor for the formation of many triterpenoids, including bacterial hopanoids and eukaryotic sterols. Triterpenoids By definition triterpenoids are triterpenes that possess heteroatoms, usually oxygen. The terms ''triterpene'' and ''triterpenoid ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Prolyl Oligopeptidase
Prolyl endopeptidase (PE) also known as prolyl oligopeptidase or post-proline cleaving enzyme is an enzyme that in humans is encoded by the ''PREP'' gene. Function Prolyl endopeptidase is a large cytosolic enzyme that belongs to a distinct class of serine peptidases. It was first described in the cytosol of rabbit brain as an oligopeptidase, which degrades the nonapeptide bradykinin at the Pro-Phe bond. The enzyme is involved in the maturation and degradation of peptide hormones and neuropeptides such as alpha-melanocyte-stimulating hormone, luteinizing hormone-releasing hormone (LH-RH), thyrotropin-releasing hormone, angiotensin, neurotensin, oxytocin, substance P and vasopressin. PREP cleaves peptide bonds at the C-terminal side of proline residues. Its activity is confined to action on oligopeptides of less than 10 kD and it has an absolute requirement for the trans-configuration of the peptide bond preceding proline. Prolyl endopeptidases are involved in the maturation a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Secondary Alcohols
In chemistry, an alcohol is a type of organic compound that carries at least one hydroxyl () functional group bound to a saturated carbon atom. The term ''alcohol'' originally referred to the primary alcohol ethanol (ethyl alcohol), which is used as a drug and is the main alcohol present in alcoholic drinks. An important class of alcohols, of which methanol and ethanol are the simplest examples, includes all compounds which conform to the general formula . Simple monoalcohols that are the subject of this article include primary (), secondary () and tertiary () alcohols. The suffix ''-ol'' appears in the IUPAC chemical name of all substances where the hydroxyl group is the functional group with the highest priority. When a higher priority group is present in the compound, the prefix ''hydroxy-'' is used in its IUPAC name. The suffix ''-ol'' in non-IUPAC names (such as paracetamol or cholesterol) also typically indicates that the substance is an alcohol. However, some compoun ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Triterpenes
Triterpenes are a class of chemical compounds composed of three terpene units with the molecular formula C30H48; they may also be thought of as consisting of six isoprene units. Animals, plants and fungi all produce triterpenes, including squalene, the precursor to all steroids. Structures Triterpenes exist in a great variety of structures. Nearly 200 different skeletons have been identified. These skeletons may be broadly divided according to the number of rings present. In general pentacyclic structures (5 rings) tend to dominate. Squalene is biosynthesized through the head-to-head condensation of two farnesyl pyrophosphate units. This coupling converts a pair of C15 components into a C30 product. Squalene serves as precursor for the formation of many triterpenoids, including bacterial hopanoids and eukaryotic sterols. Triterpenoids By definition triterpenoids are triterpenes that possess heteroatoms, usually oxygen. The terms ''triterpene'' and ''triterpenoid'' o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Betulin
Betulin is an abundant, naturally occurring triterpene. It is commonly isolated from the bark of birch trees. It forms up to 30% of the dry weight of silver birch bark. It is also found in birch sap. ''Inonotus obliquus'' and red alder also contain betulin. The compound in the bark gives the tree its white color which appears to protect the tree from mid-winter overheating by the sun. As a result, birches are some of the northernmost occurring deciduous trees. It can be converted to betulinic acid (the alcohol group replaced by a carboxylic acid group), which is biologically more active than betulin itself. History Betulin was discovered in 1788 by German-Russian chemist Johann Tobias Lowitz. Chemistry Chemically, betulin is a triterpenoid of lupane structure. It has a pentacyclic ring structure, and hydroxyl groups in positions C3 and C28. Biological activities Preliminary ''in vitro'' studies have shown that betulin demonstrates anti-cancer properties against a variety of tu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |