|
Luminophore
In chemistry, a luminophore (sometimes shortened to lumophore) is an atom or functional group in a chemical compound that is responsible for its luminescent properties. Luminophores can be either organic or inorganic. Luminophores can be further classified as fluorophores or phosphors, depending on the nature of the excited state responsible for the emission of photons. However, some luminophores cannot be classified as being exclusively fluorophores or phosphors. Examples include transition-metal complexes such as tris(bipyridine)ruthenium(II) chloride, whose luminescence comes from an excited (nominally triplet) metal-to-ligand charge-transfer (MLCT) state, which is not a true triplet state in the strict sense of the definition; and colloidal quantum dots, whose emissive state does not have either a purely singlet or triplet spin. Most luminophores consist of conjugated π systems or transition-metal complexes. There are also purely inorganic luminophores, such as zinc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phosphor
A phosphor is a substance that exhibits the phenomenon of luminescence; it emits light when exposed to some type of radiant energy. The term is used both for fluorescent or phosphorescent substances which glow on exposure to ultraviolet or visible light, and cathodoluminescent substances which glow when struck by an electron beam (cathode rays) in a cathode-ray tube. When a phosphor is exposed to radiation, the orbital electrons in its molecules are excited to a higher energy level; when they return to their former level they emit the energy as light of a certain color. Phosphors can be classified into two categories: fluorescent substances which emit the energy immediately and stop glowing when the exciting radiation is turned off, and phosphorescent substances which emit the energy after a delay, so they keep glowing after the radiation is turned off, decaying in brightness over a period of milliseconds to days. Fluorescent materials are used in applications in which the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phosphor
A phosphor is a substance that exhibits the phenomenon of luminescence; it emits light when exposed to some type of radiant energy. The term is used both for fluorescent or phosphorescent substances which glow on exposure to ultraviolet or visible light, and cathodoluminescent substances which glow when struck by an electron beam (cathode rays) in a cathode-ray tube. When a phosphor is exposed to radiation, the orbital electrons in its molecules are excited to a higher energy level; when they return to their former level they emit the energy as light of a certain color. Phosphors can be classified into two categories: fluorescent substances which emit the energy immediately and stop glowing when the exciting radiation is turned off, and phosphorescent substances which emit the energy after a delay, so they keep glowing after the radiation is turned off, decaying in brightness over a period of milliseconds to days. Fluorescent materials are used in applications in which the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemistry
Chemistry is the science, scientific study of the properties and behavior of matter. It is a natural science that covers the Chemical element, elements that make up matter to the chemical compound, compounds made of atoms, molecules and ions: their composition, structure, properties, behavior and the changes they undergo during a Chemical reaction, reaction with other Chemical substance, substances. Chemistry also addresses the nature of chemical bonds in chemical compounds. In the scope of its subject, chemistry occupies an intermediate position between physics and biology. It is sometimes called the central science because it provides a foundation for understanding both Basic research, basic and Applied science, applied scientific disciplines at a fundamental level. For example, chemistry explains aspects of plant growth (botany), the formation of igneous rocks (geology), how atmospheric ozone is formed and how environmental pollutants are degraded (ecology), the properties ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Conjugated System
In theoretical chemistry, a conjugated system is a system of connected p-orbitals with delocalized electrons in a molecule, which in general lowers the overall energy of the molecule and increases stability. It is conventionally represented as having alternating single and multiple bonds. Lone pairs, radicals or carbenium ions may be part of the system, which may be cyclic, acyclic, linear or mixed. The term "conjugated" was coined in 1899 by the German chemist Johannes Thiele. Conjugation is the overlap of one p-orbital with another across an adjacent σ bond (in transition metals, d-orbitals can be involved). A conjugated system has a region of overlapping p-orbitals, bridging the interjacent locations that simple diagrams illustrate as not having a π bond. They allow a delocalization of π electrons across all the adjacent aligned p-orbitals. The π electrons do not belong to a single bond or atom, but rather to a group of atoms. Molecules containing conjugated syst ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fluorophore
A fluorophore (or fluorochrome, similarly to a chromophore) is a fluorescent chemical compound that can re-emit light upon light excitation. Fluorophores typically contain several combined aromatic groups, or planar or cyclic molecules with several π bonds. Fluorophores are sometimes used alone, as a tracer in fluids, as a dye for staining of certain structures, as a substrate of enzymes, or as a probe or indicator (when its fluorescence is affected by environmental aspects such as polarity or ions). More generally they are covalently bonded to a macromolecule, serving as a marker (or dye, or tag, or reporter) for affine or bioactive reagents (antibodies, peptides, nucleic acids). Fluorophores are notably used to stain tissues, cells, or materials in a variety of analytical methods, i.e., fluorescent imaging and spectroscopy. Fluorescein, via its amine-reactive isothiocyanate derivative fluorescein isothiocyanate (FITC), has been one of the most popular fluorophores. Fro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chromophore
A chromophore is the part of a molecule responsible for its color. The color that is seen by our eyes is the one not absorbed by the reflecting object within a certain wavelength spectrum of visible light. The chromophore is a region in the molecule where the energy difference between two separate molecular orbitals falls within the range of the visible spectrum. Visible light that hits the chromophore can thus be absorbed by exciting an electron from its ground state into an excited state. In biological molecules that serve to capture or detect light energy, the chromophore is the moiety that causes a conformational change in the molecule when hit by light. Conjugated pi-bond system chromophores Just like how two adjacent p-orbitals in a molecule will form a pi-bond, three or more adjacent p-orbitals in a molecule can form a conjugated pi-system. In a conjugated pi-system, electrons are able to capture certain photons as the electrons resonate along a certain distance ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bioluminescence
Bioluminescence is the production and emission of light by living organisms. It is a form of chemiluminescence. Bioluminescence occurs widely in marine vertebrates and invertebrates, as well as in some fungi, microorganisms including some bioluminescent bacteria, and terrestrial arthropods such as fireflies. In some animals, the light is bacteriogenic, produced by symbiotic bacteria such as those from the genus ''Vibrio''; in others, it is autogenic, produced by the animals themselves. In a general sense, the principal chemical reaction in bioluminescence involves a light-emitting molecule and an enzyme, generally called luciferin and luciferase, respectively. Because these are generic names, luciferins and luciferases are often distinguished by the species or group, e.g. firefly luciferin. In all characterized cases, the enzyme catalyzes the oxidation of the luciferin. In some species, the luciferase requires other cofactors, such as calcium or magnesium ions, and somet ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organic Light-emitting Diode
An organic light-emitting diode (OLED or organic LED), also known as organic electroluminescent (organic EL) diode, is a light-emitting diode (LED) in which the emissive electroluminescent layer is a film of organic compound that emits light in response to an electric current. This organic layer is situated between two electrodes; typically, at least one of these electrodes is transparent. OLEDs are used to create digital displays in devices such as television screens, computer monitors, and portable systems such as smartphones and handheld game consoles. A major area of research is the development of white OLED devices for use in solid-state lighting applications. There are two main families of OLED: those based on small molecules and those employing polymers. Adding mobile ions to an OLED creates a light-emitting electrochemical cell (LEC) which has a slightly different mode of operation. An OLED display can be driven with a passive-matrix (PMOLED) or active-matrix (AMOLED) ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Zinc Orthosilicate
Willemite is a zinc silicate mineral () and a minor ore of zinc. It is highly fluorescent (green) under shortwave ultraviolet light. It occurs in a variety of colors in daylight, in fibrous masses and apple-green gemmy masses. Troostite is a variant in which part of the zinc is partly replaced by manganese, it occurs in solid brown masses. It was discovered in 1829 in the Belgian Vieille-Montagne mine. Armand Lévy (mineralogist), Armand Lévy was shown samples by a student at the university where he was teaching. Lévy named it after William I of the Netherlands (it is occasionally spelled villemite). The troostite variety is named after Dutch-American mineralogist Gerard Troost. Occurrence Willemite is usually formed as an alteration of previously existing sphalerite ore bodies, and is usually associated with limestone. It is also found in marble and may be the result of a metamorphism of earlier hemimorphite or smithsonite. Crystals have the form of hexagonal prisms terminat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Yttrium Oxide
Yttrium oxide may refer to: * Yttrium(II) oxide, YO, a dark brown solid * Yttrium(III) oxide Yttrium oxide, also known as yttria, is Y2 O3. It is an air-stable, white solid substance. The thermal conductivity of yttrium oxide is 27 W/(m·K). Uses Phosphors Yttria is widely used to make Eu:YVO4 and Eu:Y2O3 phosphors that give the red ..., Y2O3, a colorless solid {{Short pages monitor ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Rare-earth Metal
The rare-earth elements (REE), also called the rare-earth metals or (in context) rare-earth oxides or sometimes the lanthanides (yttrium and scandium are usually included as rare earths), are a set of 17 nearly-indistinguishable lustrous silvery-white soft heavy metals. Compounds containing rare earths have diverse applications in electrical and electronic components, lasers, glass, magnetic materials, and industrial processes. Scandium and yttrium are considered rare-earth elements because they tend to occur in the same ore deposits as the lanthanides and exhibit similar chemical properties, but have different electronic and magnetic properties. These metals tarnish slowly in air at room temperature and react slowly with cold water to form hydroxides, liberating hydrogen. They react with steam to form oxides, and at elevated temperature (400°C) ignite spontaneously. These elements and their compounds have no biological function other than in several specialized enzymes, su ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
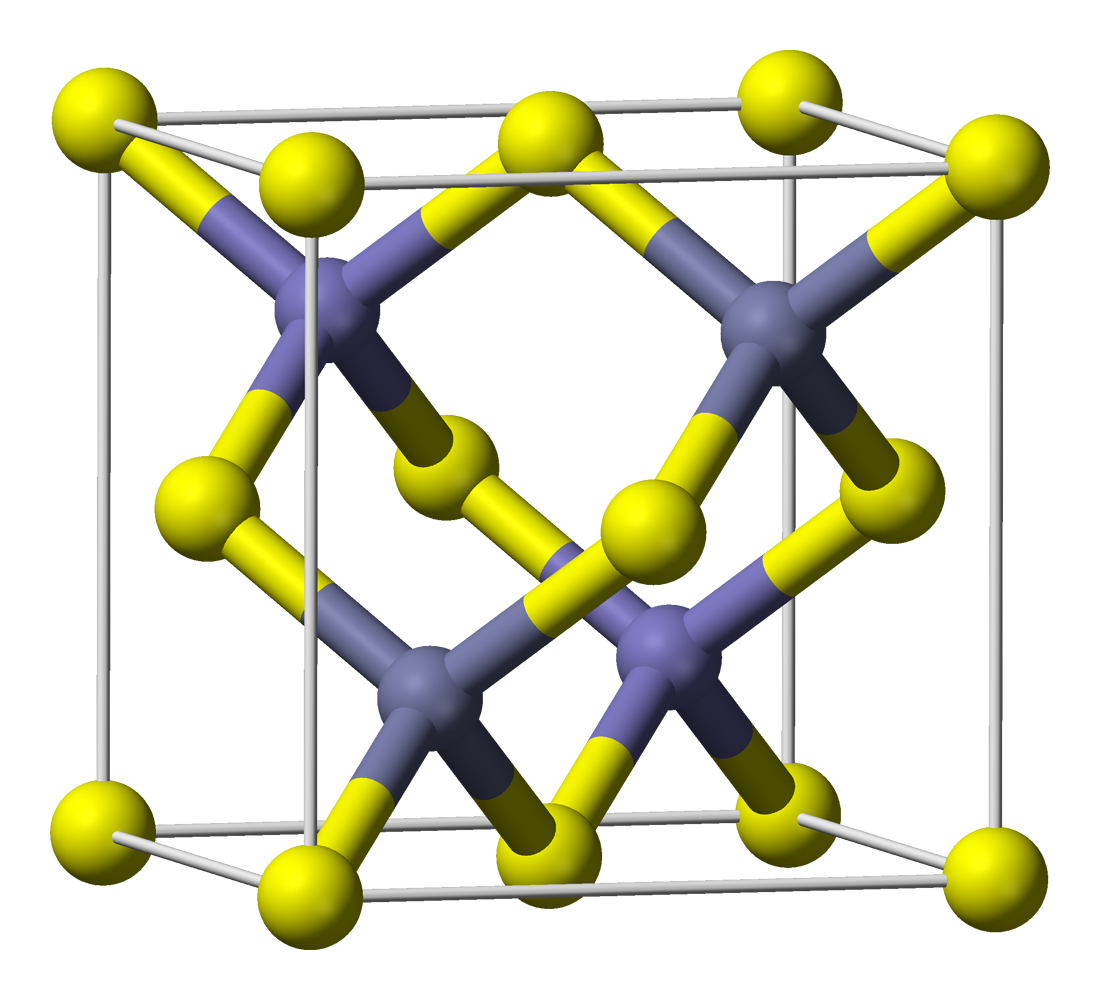
Zinc Sulfide
Zinc sulfide (or zinc sulphide) is an inorganic compound with the chemical formula of ZnS. This is the main form of zinc found in nature, where it mainly occurs as the mineral sphalerite. Although this mineral is usually black because of various impurities, the pure material is white, and it is widely used as a pigment. In its dense synthetic form, zinc sulfide can be transparent, and it is used as a window for visible optics and infrared optics. Structure ZnS exists in two main crystalline forms. This dualism is an example of polymorphism. In each form, the coordination geometry at Zn and S is tetrahedral. The more stable cubic form is known also as zinc blende or sphalerite. The hexagonal form is known as the mineral wurtzite, although it also can be produced synthetically.. The transition from the sphalerite form to the wurtzite form occurs at around 1020 °C. A tetragonal form is also known as the very rare mineral called polhemusite, with the formula . Applicatio ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |



.jpeg/1200px-Fall_Leaves_(199582361).jpeg)