|
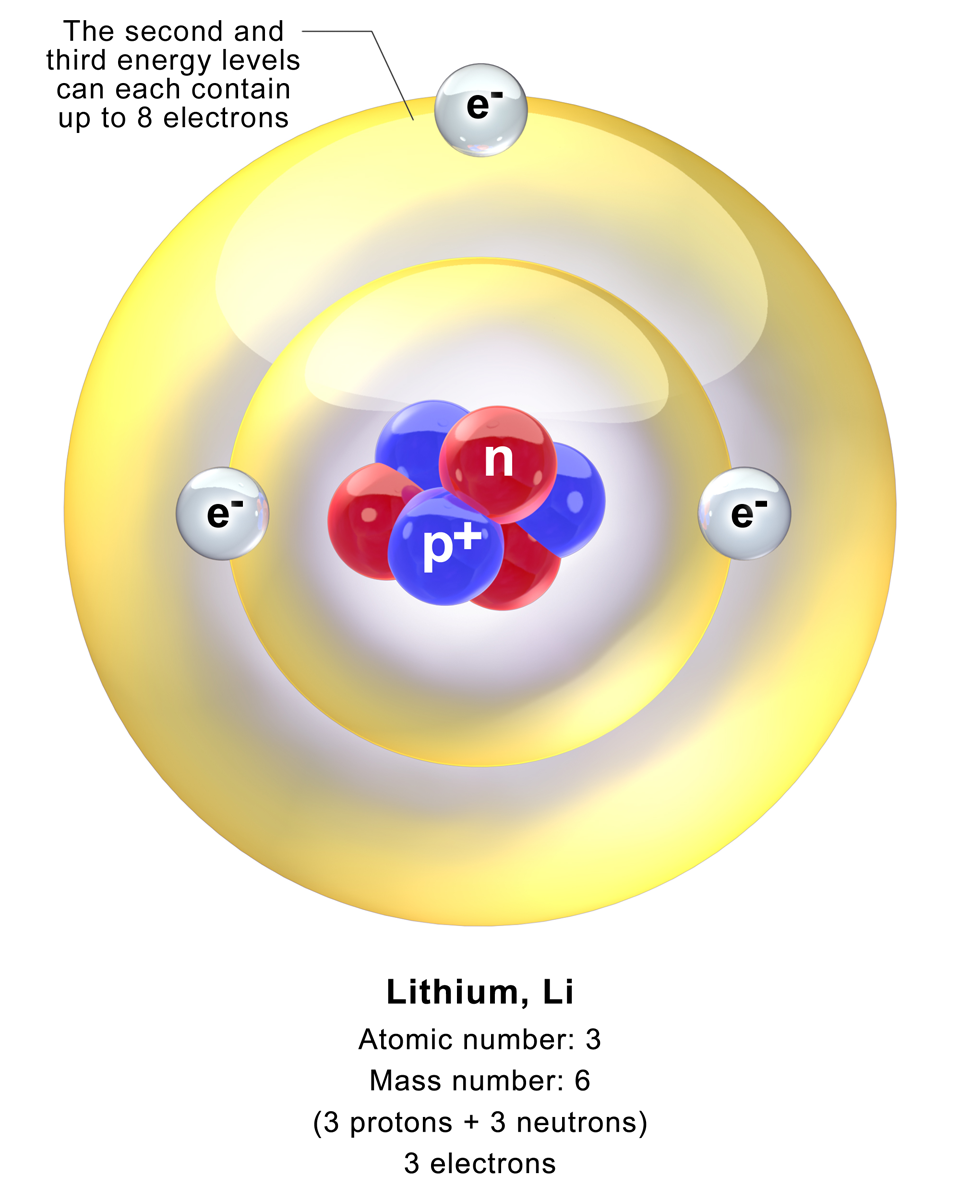
Lithium Atom
A lithium atom is an atom of the chemical element lithium. Stable lithium is composed of three electrons bound by the electromagnetic force to a nucleus containing three protons along with either three or four neutrons, depending on the isotope, held together by the strong force. Similarly to the case of the helium atom, a closed-form solution to the Schrödinger equation for the lithium atom has not been found. However, various approximations, such as the Hartree–Fock method, can be used to estimate the ground state energy and wavefunction of the atom. The quantum defect is a value that describes the deviation from hydrogenic A hydrogen-like atom (or hydrogenic atom) is any atom or ion with a single valence electron. These atoms are isoelectronic with hydrogen. Examples of hydrogen-like atoms include, but are not limited to, hydrogen itself, all alkali metals such a ... energy levels. Further reading W. Zheng et al. / Appl. Math. Comput. 153 (2004) 685–695"Numerical solu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Blausen 0615 Lithium Atom
Blausen Medical Communications, Inc. is the creator and owner of a library of two- and three-dimensional medical and scientific images and animations, a developer of information technology allowing access to that content, and a business focused on licensing and distributing the content. It was founded by Bruce Blausen in Houston, Texas, in 1991, and is privately held. Background Blausen Medical Communications, Inc. (BMC) is a privately held company founded by Bruce Blausen in Houston, Texas in 1991. BMC created and owns a library of medical and scientific images and animations, and has developed information technology tools allowing access to the library; as well, it licenses and otherwise works to distribute the content. As of this date, BMC's animation library comprised approximately 1,500 animations and over 27,000 two- and three-dimensional images designed for point-of-care patient education, which could be accessed by consumers or professional caregivers (primarily via h ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Helium Atom
A helium atom is an atom of the chemical element helium. Helium is composed of two electrons bound by the electromagnetic force to a nucleus containing two protons along with either one or two neutrons, depending on the isotope, held together by the strong force. Unlike for hydrogen, a closed-form solution to the Schrödinger equation for the helium atom has not been found. However, various approximations, such as the Hartree–Fock method, can be used to estimate the ground state energy and wavefunction of the atom. Introduction The quantum mechanical description of the helium atom is of special interest, because it is the simplest multi-electron system and can be used to understand the concept of quantum entanglement. The Hamiltonian of helium, considered as a three-body system of two electrons and a nucleus and after separating out the centre-of-mass motion, can be written as H(\mathbf_1,\, \mathbf_2) = \sum_\left(-\frac \nabla^2_ -\frac\right) - \frac \nabla_ \cdot \nabla_ ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrogenic
A hydrogen-like atom (or hydrogenic atom) is any atom or ion with a single valence electron. These atoms are isoelectronic with hydrogen. Examples of hydrogen-like atoms include, but are not limited to, hydrogen itself, all alkali metals such as Rb and Cs, singly ionized alkaline earth metals such as Ca+ and Sr+ and other ions such as He+, Li2+, and Be3+ and isotopes of any of the above. A hydrogen-like atom includes a positively charged core consisting of the atomic nucleus and any core electrons as well as a single valence electron. Because helium is common in the universe, the spectroscopy of singly ionized helium is important in EUV astronomy, for example, of DO white dwarf stars. The non-relativistic Schrödinger equation and relativistic Dirac equation for the hydrogen atom can be solved analytically, owing to the simplicity of the two-particle physical system. The one-electron wave function solutions are referred to as ''hydrogen-like atomic orbitals''. Hydrogen-li ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Quantum Defect
The term quantum defect refers to two concepts: energy loss in lasers and energy levels in alkali elements. Both deal with quantum systems where matter interacts with light. In laser science In laser science, the term "quantum defect" refers to the fact that the energy of a pump photon is generally higher than that of a ''signal photon'' (photon of the output radiation). The energy difference is lost to heat, which may carry away the excess entropy delivered by the multimode incoherent pump. The quantum defect of a laser can be defined as part of the energy of the pumping photon, which is lost (not turned into photons at the lasing wavelength) in the gain medium at the lasing. At given frequency \omega_ of pump and given frequency \omega_ of lasing, the quantum defect q = \hbar \omega_ - \hbar\omega_. Such quantum defect has dimension of energy; for the efficient operation, the temperature of the gain medium (measured in units of energy) should be small compared to the quantum def ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Wavefunction
A wave function in quantum physics is a mathematical description of the quantum state of an isolated quantum system. The wave function is a complex-valued probability amplitude, and the probabilities for the possible results of measurements made on the system can be derived from it. The most common symbols for a wave function are the Greek letters and (lower-case and capital psi, respectively). The wave function is a function of the degrees of freedom corresponding to some maximal set of commuting observables. Once such a representation is chosen, the wave function can be derived from the quantum state. For a given system, the choice of which commuting degrees of freedom to use is not unique, and correspondingly the domain of the wave function is also not unique. For instance, it may be taken to be a function of all the position coordinates of the particles over position space, or the momenta of all the particles over momentum space; the two are related by a Fourier trans ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ground State
The ground state of a quantum-mechanical system is its stationary state of lowest energy; the energy of the ground state is known as the zero-point energy of the system. An excited state is any state with energy greater than the ground state. In quantum field theory, the ground state is usually called the vacuum state or the vacuum. If more than one ground state exists, they are said to be degenerate. Many systems have degenerate ground states. Degeneracy occurs whenever there exists a unitary operator that acts non-trivially on a ground state and commutes with the Hamiltonian of the system. According to the third law of thermodynamics, a system at absolute zero temperature exists in its ground state; thus, its entropy is determined by the degeneracy of the ground state. Many systems, such as a perfect crystal lattice, have a unique ground state and therefore have zero entropy at absolute zero. It is also possible for the highest excited state to have absolute zero temper ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hartree–Fock Method
In computational physics and chemistry, the Hartree–Fock (HF) method is a method of approximation for the determination of the wave function and the energy of a quantum many-body system in a stationary state. The Hartree–Fock method often assumes that the exact ''N''-body wave function of the system can be approximated by a single Slater determinant (in the case where the particles are fermions) or by a single permanent (in the case of bosons) of ''N'' spin-orbitals. By invoking the variational method, one can derive a set of ''N''-coupled equations for the ''N'' spin orbitals. A solution of these equations yields the Hartree–Fock wave function and energy of the system. Especially in the older literature, the Hartree–Fock method is also called the self-consistent field method (SCF). In deriving what is now called the Hartree equation as an approximate solution of the Schrödinger equation, Hartree required the final field as computed from the charge distribution to be "s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Schrödinger Equation
The Schrödinger equation is a linear partial differential equation that governs the wave function of a quantum-mechanical system. It is a key result in quantum mechanics, and its discovery was a significant landmark in the development of the subject. The equation is named after Erwin Schrödinger, who postulated the equation in 1925, and published it in 1926, forming the basis for the work that resulted in his Nobel Prize in Physics in 1933. Conceptually, the Schrödinger equation is the quantum counterpart of Newton's second law in classical mechanics. Given a set of known initial conditions, Newton's second law makes a mathematical prediction as to what path a given physical system will take over time. The Schrödinger equation gives the evolution over time of a wave function, the quantum-mechanical characterization of an isolated physical system. The equation can be derived from the fact that the time-evolution operator must be unitary, and must therefore be generated by t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Strong Force
The strong interaction or strong force is a fundamental interaction that confines quarks into proton, neutron, and other hadron particles. The strong interaction also binds neutrons and protons to create atomic nuclei, where it is called the nuclear force. Most of the mass of a common proton or neutron is the result of the strong interaction energy; the individual quarks provide only about 1% of the mass of a proton. At the range of 10−15 m (slightly more than the radius of a nucleon), the strong force is approximately 100 times as strong as electromagnetism, 106 times as strong as the weak interaction, and 1038 times as strong as gravitation. The strong interaction is observable at two ranges and mediated by two force carriers. On a larger scale (of about 1 to 3 fm), it is the force (carried by mesons) that binds protons and neutrons (nucleons) together to form the nucleus of an atom. On the smaller scale (less than about 0.8 fm, the radius of a nucleon), it ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
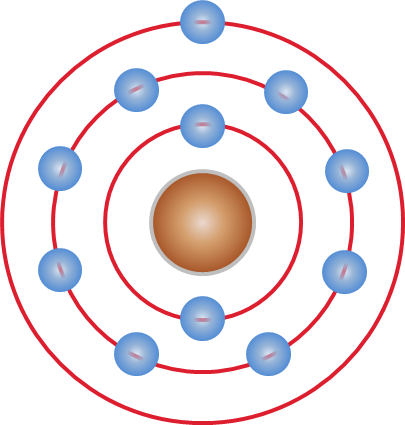
Atom
Every atom is composed of a nucleus and one or more electrons bound to the nucleus. The nucleus is made of one or more protons and a number of neutrons. Only the most common variety of hydrogen has no neutrons. Every solid, liquid, gas, and plasma is composed of neutral or ionized atoms. Atoms are extremely small, typically around 100 picometers across. They are so small that accurately predicting their behavior using classical physics, as if they were tennis balls for example, is not possible due to quantum effects. More than 99.94% of an atom's mass is in the nucleus. The protons have a positive electric charge, the electrons have a negative electric charge, and the neutrons have no electric charge. If the number of protons and electrons are equal, then the atom is electrically neutral. If an atom has more or fewer electrons than protons, then it has an overall negative or positive charge, respectively – such atoms are called ions. The electrons of an atom are a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isotopes Of Lithium
Naturally occurring lithium (3Li) is composed of two stable isotopes, lithium-6 and lithium-7, with the latter being far more abundant on Earth. Both of the natural isotopes have an unexpectedly low nuclear binding energy per nucleon ( for lithium-6 and for lithium-7) when compared with the adjacent lighter and heavier elements, helium ( for helium-4) and beryllium ( for beryllium-9). The longest-lived radioisotope of lithium is lithium-8, which has a half-life of just . Lithium-9 has a half-life of , and lithium-11 has a half-life of . All of the remaining isotopes of lithium have half-lives that are shorter than 10 nanoseconds. The shortest-lived known isotope of lithium is lithium-4, which decays by proton emission with a half-life of about (), although the half-life of lithium-3 is yet to be determined, and is likely to be much shorter, like helium-2 (diproton) which undergoes proton emission within s. Lithium-7 and lithium-6 are two of the primordial nuclides that wer ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Neutron
The neutron is a subatomic particle, symbol or , which has a neutral (not positive or negative) charge, and a mass slightly greater than that of a proton. Protons and neutrons constitute the nuclei of atoms. Since protons and neutrons behave similarly within the nucleus, and each has a mass of approximately one atomic mass unit, they are both referred to as nucleons. Their properties and interactions are described by nuclear physics. Protons and neutrons are not elementary particles; each is composed of three quarks. The chemical properties of an atom are mostly determined by the configuration of electrons that orbit the atom's heavy nucleus. The electron configuration is determined by the charge of the nucleus, which is determined by the number of protons, or atomic number. The number of neutrons is the neutron number. Neutrons do not affect the electron configuration, but the sum of atomic and neutron numbers is the mass of the nucleus. Atoms of a chemical element t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |



.png)
