|
Lithium Metal Battery
Lithium metal batteries are primary batteries that have metallic lithium as an anode. These types of batteries are also referred to as lithium-metal batteries after lithium-ion batteries had been invented. Most lithium metal batteries are non-rechargeable. However, rechargeable lithium metal batteries are also under development. Since 2007, Dangerous Goods Regulations differentiate between lithium metal batteries (UN 3090) and lithium-ion batteries (UN 3480). They stand apart from other batteries in their high charge density and high cost per unit. Depending on the design and chemical compounds used, lithium cells can produce voltages from (comparable to a zinc–carbon or alkaline battery) to about . Disposable primary lithium batteries must be distinguished from secondary lithium-ion or a lithium-polymer, which are rechargeable batteries and contain no metallic lithium. Lithium is especially useful, because its ions can be arranged to move between the anode and the ca ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electrolytes
An electrolyte is a medium containing ions that is electrically conducting through the movement of those ions, but not conducting electrons. This includes most soluble salts, acids, and bases dissolved in a polar solvent, such as water. Upon dissolving, the substance separates into cations and anions, which disperse uniformly throughout the solvent. Solid-state electrolytes also exist. In medicine and sometimes in chemistry, the term electrolyte refers to the substance that is dissolved. Electrically, such a solution is neutral. If an electric potential is applied to such a solution, the cations of the solution are drawn to the electrode that has an abundance of electrons, while the anions are drawn to the electrode that has a deficit of electrons. The movement of anions and cations in opposite directions within the solution amounts to a current. Some gases, such as hydrogen chloride (HCl), under conditions of high temperature or low pressure can also function as electrolytes. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lithium Tetrafluoroborate
Lithium tetrafluoroborate is an inorganic compound with the formula Li BF4. It is a white crystalline powder. It has been extensively tested for use in commercial secondary batteries, an application that exploits its high solubility in nonpolar solvents.Xu, Kang. "Nonaqueous Liquid Electrolytes for Lithium-Based Rechargeable Batteries."Chemical Reviews 2004, volume 104, pp. 4303-418. Applications Although BF4− has high ionic mobility, solutions of its Li+ salt are less conductive than other less associated salts. As an electrolyte in lithium-ion batteries, LiBF4 offers some advantages relative to the more common LiPF6. It exhibits greater thermal stability and moisture tolerance. For example, LiBF4 can tolerate a moisture content up to 620 ppm at room temperature whereas LiPF6 readily hydrolyzes into toxic POF3 and HF gases, often destroying the battery's electrode materials. Disadvantages of the electrolyte include a relatively low conductivity and difficulties form ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbon Monofluoride
Carbon monofluoride (CF, CFx, or (CF)n), also called polycarbon monofluoride (PMF), polycarbon fluoride, poly(carbon monofluoride), and graphite fluoride, is a material formed by high-temperature reaction of fluorine gas with graphite, charcoal, or pyrolytic carbon powder. It is a highly hydrophobic microcrystalline powder. Its CAS number is . In contrast to graphite intercalation compounds it is a covalent graphite compound. Carbon is stable in a fluorine atmosphere up to about 400 °C, but between 420-600 °C a reaction takes place to give substoichiometric carbon monofluoride, CF0.68 appearing dark grey. With increasing temperature and fluorine pressure stoichiometries up to CF1.12 are formed. With increasing fluorine content the colour changes from dark grey to cream white indicating the loss of the aromatic character. The fluorine atoms are located in an alternating fashion above and under the former graphene plane, which is now buckled due to formation of covalent ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Substance Of Very High Concern
A substance of very high concern (SVHC) is a chemical substance (or part of a group of chemical substances) concerning which it has been proposed that use within the European Union be subject to authorisation under the REACH Regulation. Indeed, listing of a substance as an SVHC by the European Chemicals Agency (ECHA) is the first step in the procedure for authorisation or restriction of use of a chemical. The first list of SVHCs was published on 28 October 2008 and the list has been updated many times to include new candidates. The most recent update occurred in January 2022 to include a total of 223 SVHC. Criteria The criteria are given in article 57 of the REACH Regulation. A substance ''may'' be proposed as an SVHC if it meets one or more of the following criteria: *it is carcinogenic; *it is mutagenic; *it is toxic for reproduction; *it is persistent, bioaccumulative and toxicAnnex XIII, REACH Regulation, at pp. 383–85. (PBT substances); *it is very persistent and very b ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
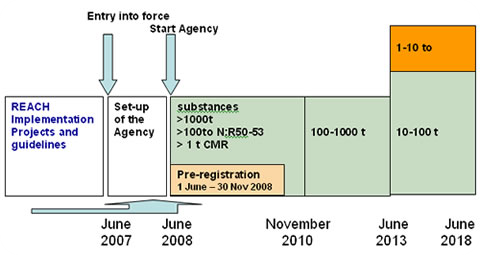
Registration, Evaluation, Authorisation And Restriction Of Chemicals
Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH) is a European Union regulation dating from 18 December 2006. REACH addresses the production and use of chemical substances, and their potential impacts on both human health and the environment. Its 849 pages took seven years to pass, and it has been described as the most complex legislation in the Union's history and the most important in 20 years. It is the strictest law to date regulating chemical substances and will affect industries throughout the world. REACH entered into force on 1 June 2007, with a phased implementation over the next decade. The regulation also established the European Chemicals Agency, which manages the technical, scientific and administrative aspects of REACH. Overview When REACH is fully in force, it will require all companies manufacturing or importing chemical substances into the European Union in quantities of one tonne or more per year to register these substances with a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
1,2-dimethoxyethane
Dimethoxyethane, also known as glyme, monoglyme, dimethyl glycol, ethylene glycol dimethyl ether, dimethyl cellosolve, and DME, is a colorless, aprotic, and liquid ether that is used as a solvent, especially in batteries. Dimethoxyethane is miscible with water. Production Monoglyme is produced industrially by the reaction of dimethylether with ethylene oxide: :CH3OCH3 + CH2CH2O → CH3OCH2CH2OCH3 Applications as solvent and ligand left, 144px, Structure of the coordination complex NbCl3(dimethoxyethane)(3-hexyne).{{cite journal , doi=10.1021/ja8100837, title=New Tantalum Ligand-Free Catalyst System for Highly Selective Trimerization of Ethylene Affording 1-Hexene: New Evidence of a Metallacycle Mechanism, year=2009, last1=Arteaga-Müller, first1=Rocío, last2=Tsurugi, first2=Hayato, last3=Saito, first3=Teruhiko, last4=Yanagawa, first4=Masao, last5=Oda, first5=Seiji, last6=Mashima, first6=Kazushi, journal=Journal of the American Chemical Society, volume=131, issue=15, pages=5370 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dimethoxyethane
Dimethoxyethane, also known as glyme, monoglyme, dimethyl glycol, ethylene glycol dimethyl ether, dimethyl cellosolve, and DME, is a colorless, aprotic, and liquid ether that is used as a solvent, especially in batteries. Dimethoxyethane is miscible with water. Production Monoglyme is produced industrially by the reaction of dimethylether with ethylene oxide: :CH3OCH3 + CH2CH2O → CH3OCH2CH2OCH3 Applications as solvent and ligand left, 144px, Structure of the coordination complex NbCl3(dimethoxyethane)(3-hexyne).{{cite journal , doi=10.1021/ja8100837, title=New Tantalum Ligand-Free Catalyst System for Highly Selective Trimerization of Ethylene Affording 1-Hexene: New Evidence of a Metallacycle Mechanism, year=2009, last1=Arteaga-Müller, first1=Rocío, last2=Tsurugi, first2=Hayato, last3=Saito, first3=Teruhiko, last4=Yanagawa, first4=Masao, last5=Oda, first5=Seiji, last6=Mashima, first6=Kazushi, journal=Journal of the American Chemical Society, volume=131, issue=15, pages=53 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Propylene Carbonate
Propylene carbonate (often abbreviated PC) is an organic compound with the formula C4H6O3. It is a cyclic carbonate ester derived from propylene glycol. This colorless and odorless liquid is useful as a polar, aprotic solvent. Propylene carbonate is chiral, but is used as the racemic mixture in most contexts. Preparation Although many organic carbonates are produced using phosgene, propylene and ethylene carbonates are exceptions. They are mainly prepared by the carbonation of the epoxides (epoxypropane, or propylene oxide here): :CH3CHCH2O + CO2 → CH3C2H3O2CO The process is particularly attractive since the production of these epoxides consumes carbon dioxide. Thus this reaction is a good example of a green process. The corresponding reaction of 1,2-propanediol with phosgene is complex, yielding not only propylene carbonate but also oligomeric products. Propylene carbonate can also be synthesized from urea and propylene glycol over zinc acetate. Applications ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lithium Perchlorate
Lithium perchlorate is the inorganic compound with the formula LiClO4. This white or colourless crystalline salt is noteworthy for its high solubility in many solvents. It exists both in anhydrous form and as a trihydrate. Applications Inorganic chemistry Lithium perchlorate is used as a source of oxygen in some chemical oxygen generators. It decomposes at about 400 °C, yielding lithium chloride and oxygen: : LiClO4 → LiCl + 2 O2 Over 60% of the mass of the lithium perchlorate is released as oxygen. It has both the highest oxygen to weight and oxygen to volume ratio of all practical perchlorate salts. Organic chemistry LiClO4 is highly soluble in organic solvents, even diethyl ether. Such solutions are employed in Diels–Alder reactions, where it is proposed that the Lewis acidic Li+ binds to Lewis basic sites on the dienophile, thereby accelerating the reaction. Lithium perchlorate is also used as a co-catalyst in the coupling of α,β-unsaturated carbonyls ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Wh/kg
The watt-hour per kilogram ( SI symbol: W⋅h/kg) is a unit of specific energy commonly used to measure the density of energy in batteries and capacitors. SI Units In the SI system of measurement, one watt-hour per kilogram is equal to 3600 joules per kilogram. Typical values The batteries that Tesla uses in their electric cars deliver about 254 W⋅h/kg, compared to supercapacitors that are typically rated between 3–10 W⋅h/kg, with the best commercially available supercapacitors as high as 47 W⋅h/kg. Nuclear batteries based on betavoltaics A betavoltaic device (betavoltaic cell or betavoltaic battery) is a type of nuclear battery which generates electric current from beta particles (electrons) emitted from a radioactive source, using semiconductor junctions. A common source used is ... can reach up to 3300 W⋅h/kg, although over much longer time periods. [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |