|
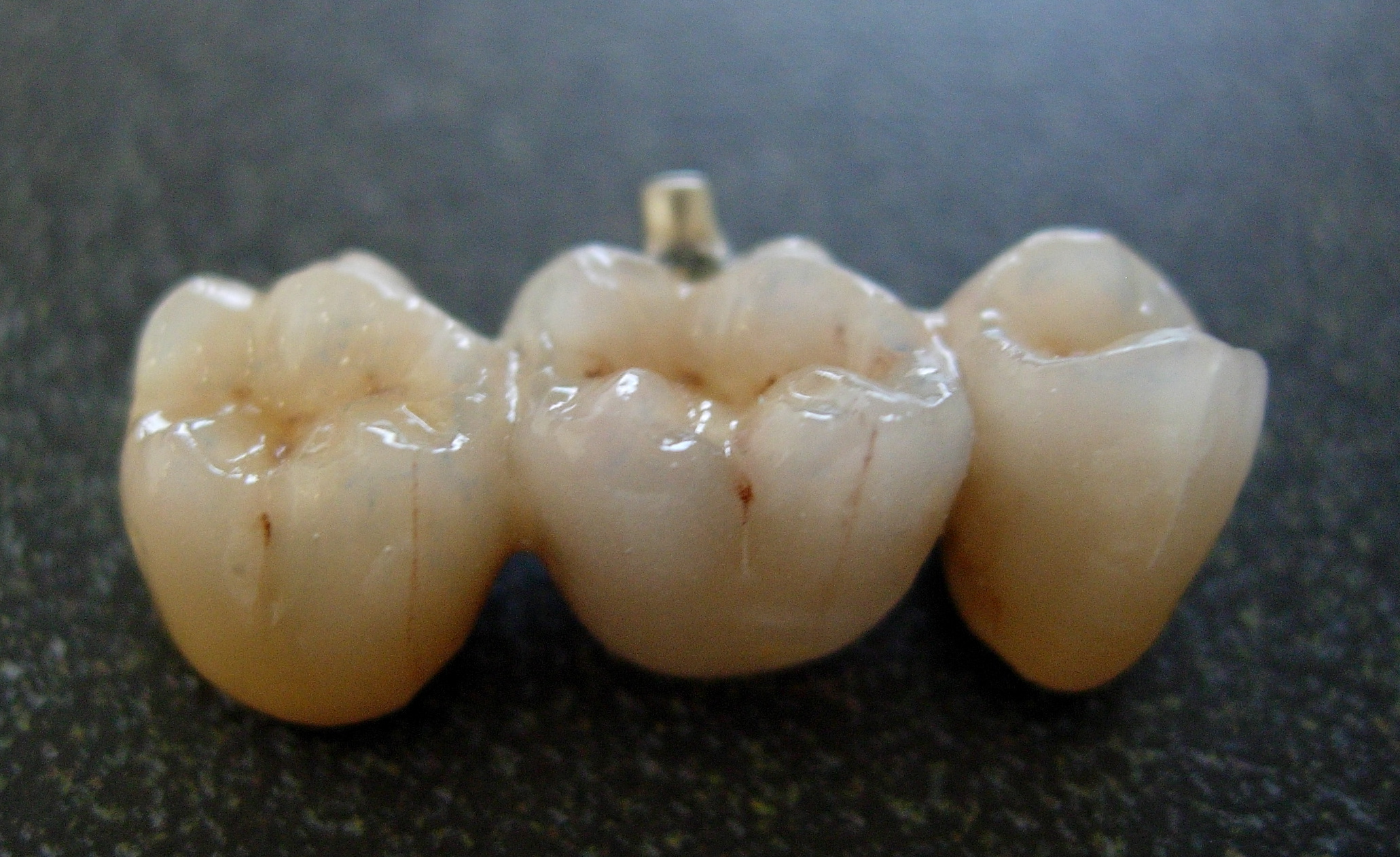
Lithium Disilicate
Lithium disilicate (Li2Si2O5) is a chemical compound that is a glass ceramic. It is widely used as a dental ceramic due to its strength, machinability and translucency. Use Lithium disilicate has found applications in dentistry as a dental ceramic material for dental restorations such as Crown (dentistry), crowns, Bridge (dentistry), bridges, and Veneer (dentistry), veneers in the form of Li2Si2O5. Lithium disilicate has an unusual microstructure that consists of many randomly oriented small and interlocking plate-like needle-like crystals. This structure causes cracks to be deflected, blunted, and/or to branch, which prevents cracks from growing. Lithium disilicate has a biaxial flexible strength in the range of 360 MPa to 400 MPa; in comparison, for metal ceramics this is around 80 to 100 MPa, for wikt:Special:Search/veneer, veneered zirconia it is approximately 100 MPa, and for leucite glass ceramic it is approximately 150 to 160 MPa. It has high hardness (5.92 +/- 0.18 GPa) ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lithium
Lithium (from el, λίθος, lithos, lit=stone) is a chemical element with the symbol Li and atomic number 3. It is a soft, silvery-white alkali metal. Under standard conditions, it is the least dense metal and the least dense solid element. Like all alkali metals, lithium is highly reactive and flammable, and must be stored in vacuum, inert atmosphere, or inert liquid such as purified kerosene or mineral oil. When cut, it exhibits a metallic luster, but moist air corrodes it quickly to a dull silvery gray, then black tarnish. It never occurs freely in nature, but only in (usually ionic) compounds, such as pegmatitic minerals, which were once the main source of lithium. Due to its solubility as an ion, it is present in ocean water and is commonly obtained from brines. Lithium metal is isolated electrolytically from a mixture of lithium chloride and potassium chloride. The nucleus of the lithium atom verges on instability, since the two stable lithium isotopes foun ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Silicate
In chemistry, a silicate is any member of a family of polyatomic anions consisting of silicon and oxygen, usually with the general formula , where . The family includes orthosilicate (), metasilicate (), and pyrosilicate (, ). The name is also used for any salt of such anions, such as sodium metasilicate; or any ester containing the corresponding chemical group, such as tetramethyl orthosilicate. The name "silicate" is sometimes extended to any anions containing silicon, even if they do not fit the general formula or contain other atoms besides oxygen; such as hexafluorosilicate .Most commonly, silicates are encountered as silicate minerals. For diverse manufacturing, technological, and artistic needs, silicates are versatile materials, both natural (such as granite, gravel, and garnet) and artificial (such as Portland cement, ceramics, glass, and waterglass). Structural principles In all silicates, silicon atom occupies the center of an idealized tetrahedron whose corner ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Compound
A chemical compound is a chemical substance composed of many identical molecules (or molecular entities) containing atoms from more than one chemical element held together by chemical bonds. A molecule consisting of atoms of only one element is therefore not a compound. A compound can be transformed into a different substance by a chemical reaction, which may involve interactions with other substances. In this process, bonds between atoms may be broken and/or new bonds formed. There are four major types of compounds, distinguished by how the constituent atoms are bonded together. Molecular compounds are held together by covalent bonds; ionic compounds are held together by ionic bonds; intermetallic compounds are held together by metallic bonds; coordination complexes are held together by coordinate covalent bonds. Non-stoichiometric compounds form a disputed marginal case. A chemical formula specifies the number of atoms of each element in a compound molecule, using the s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dental Ceramic
Dental porcelain (also known as dental ceramic) is a dental material used by dental technicians to create biocompatible lifelike dental restorations, such as crowns, bridges, and veneers. Evidence suggests they are an effective material as they are biocompatible, aesthetic, insoluble and have a hardness of 7 on the Mohs scale. For certain dental prostheses, such as three-unit molars porcelain fused to metal or in complete porcelain group, zirconia-based restorations are recommended. The word "ceramic" is derived from the Greek word ''keramos'', meaning "potter's clay". It came from the ancient art of fabricating pottery where mostly clay was fired to form a hard, brittle object; a more modern definition is a material that contains metallic and non-metallic elements (usually oxygen). These materials can be defined by their inherent properties including their hard, stiff, and brittle nature due to the structure of their inter-atomic bonding, which is both ionic and covalent. In cont ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dentistry
Dentistry, also known as dental medicine and oral medicine, is the branch of medicine focused on the teeth, gums, and mouth. It consists of the study, diagnosis, prevention, management, and treatment of diseases, disorders, and conditions of the mouth, most commonly focused on dentition (the development and arrangement of teeth) as well as the oral mucosa. Dentistry may also encompass other aspects of the craniofacial complex including the temporomandibular joint. The practitioner is called a dentist. The history of dentistry is almost as ancient as the history of humanity and civilization with the earliest evidence dating from 7000 BC to 5500 BC. Dentistry is thought to have been the first specialization in medicine which have gone on to develop its own accredited degree with its own specializations. Dentistry is often also understood to subsume the now largely defunct medical specialty of stomatology (the study of the mouth and its disorders and diseases) for which reas ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Crown (dentistry)
In dentistry, a crown most commonly refers to a dental cap, a type of dental restoration that completely caps or encircles a tooth or dental implant. A crown may be needed when a large cavity threatens the health of a tooth. A crown is typically bonded to the tooth by dental cement. They can be made from various materials, which are usually fabricated using ''indirect methods''. Crowns are used to improve the strength or appearance of teeth and to halt deterioration. While beneficial to dental health, the procedure and materials can be costly. The most common method of crowning a tooth involves taking a dental impression of a tooth prepared by a dentist, then fabricating the crown outside of the mouth. The crown can then be inserted at a subsequent dental appointment. This ''indirect method'' of tooth restoration allows use of strong restorative material requiring time-consuming fabrication under intense heat, such as casting metal or firing porcelain, that would not be possib ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bridge (dentistry)
A bridge is a fixed dental restoration (a fixed dental prosthesis) used to replace one or more missing teeth by joining an artificial tooth definitively to adjacent teeth or dental implants. Definitions Fixed bridge: A dental prosthesis that is definitively attached to natural teeth and replaces missing teeth. Abutment: The tooth that supports and retains a dental prosthesis. Pontic: The artificial tooth that replaces a missing natural tooth. Retainer: The component attached to the abutment for retention of the prosthesis. Retainers can be major or minor. Unit: Pontics and abutment teeth are referred to as units. The total number of units in a bridge is equal to the number of pontics plus the number of abutment teeth. Saddle: The area on the alveolar ridge which is edentulous where at least one missing tooth is to be reinstated. Connector: Joins the pontic to the retainer or two retainers together. Connectors may be fixed or movable. Span: The length of the alveolar rid ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Veneer (dentistry)
In dentistry, a veneer is a layer of material placed over a tooth. Veneers can improve the aesthetics of a smile and protect the tooth's surface from damage. There are two main types of material used to fabricate a veneer: composite and dental porcelain. A composite veneer may be directly placed (built-up in the mouth), or indirectly fabricated by a dental technician in a dental lab, and later bonded to the tooth, typically using a resin cement. They are typically used for treatment of adolescent patients who will require a more permanent design once they are fully grown. The lifespan of a composite veneer is approximately four years. In contrast, a porcelain veneer may only be indirectly fabricated. A full veneer crown is described as "a restoration that covers all the coronal tooth surfaces (mesial, distal, facial, lingual and occlusal)". Laminate veneer, on the other hand, is a thin layer that covers only the surface of the tooth and is generally used for aesthetic purposes. Th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Metal Ceramics
A metal (from Greek μέταλλον ''métallon'', "mine, quarry, metal") is a material that, when freshly prepared, polished, or fractured, shows a lustrous appearance, and conducts electricity and heat relatively well. Metals are typically ductile (can be drawn into wires) and malleable (they can be hammered into thin sheets). These properties are the result of the ''metallic bond'' between the atoms or molecules of the metal. A metal may be a chemical element such as iron; an alloy such as stainless steel; or a molecular compound such as polymeric sulfur nitride. In physics, a metal is generally regarded as any substance capable of conducting electricity at a temperature of absolute zero. Many elements and compounds that are not normally classified as metals become metallic under high pressures. For example, the nonmetal iodine gradually becomes a metal at a pressure of between 40 and 170 thousand times atmospheric pressure. Equally, some materials regarded as metals ca ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Zirconia
Zirconium dioxide (), sometimes known as zirconia (not to be confused with zircon), is a white crystalline oxide of zirconium. Its most naturally occurring form, with a monoclinic crystalline structure, is the mineral baddeleyite. A dopant stabilized cubic structured zirconia, cubic zirconia, is synthesized in various colours for use as a gemstone and a diamond simulant. Production, chemical properties, occurrence Zirconia is produced by calcining zirconium compounds, exploiting its high thermostability.Ralph Nielsen "Zirconium and Zirconium Compounds" in Ullmann's Encyclopedia of Industrial Chemistry, 2005, Wiley-VCH, Weinheim. Structure Three phases are known: monoclinic below 1170 °C, tetragonal between 1170 °C and 2370 °C, and cubic above 2370 °C. The trend is for higher symmetry at higher temperatures, as is usually the case. A small percentage of the oxides of calcium or yttrium stabilize in the cubic phase. The very rare mineral tazheranite, , ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glass Ceramic
Glass-ceramics are polycrystalline materials produced through controlled crystallization of base glass, producing a fine uniform dispersion of crystals throughout the bulk material. Crystallization is accomplished by subjecting suitable glasses to a carefully regulated heat treatment schedule, resulting in the nucleation and growth of crystal phases. In many cases, the crystallization process can proceed to near completion, but in a small proportion of processes, the residual glass phase often remains. Glass-ceramic materials share many properties with both glasses and ceramics. Glass-ceramics have an amorphous phase and one or more crystalline phases and are produced by a so-called "controlled crystallization" in contrast to a spontaneous crystallization, which is usually not wanted in glass manufacturing. Glass-ceramics have the fabrication advantage of glass, as well as special properties of ceramics. When used for sealing, some glass-ceramics do not require brazing but can with ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |