|
List Of Things Named After Johannes Rydberg
{{Short description, none Janne Rydberg was a Swedish spectroscopist and physicist, whom the following are named after: * Rydberg constant **Rydberg, a unit of energy, derived from the Rydberg constant, equal to half the Hartree energy * Rydberg correction * Rydberg formula * Rydberg ionization spectroscopy * Rydberg state **Heavy Rydberg system ** Rydberg atom ** Rydberg matter **Rydberg molecule ** Rydberg polaron * Rydberg–Klein–Rees method *Rydberg–Ritz combination principle Others * Mendel-Rydberg Basin *Rydberg (crater) Rydberg is a lunar impact crater that is located on the far side of the Moon, just past the southwest limb. It lies due south of the Mare Orientale, in the outer skirt of ejecta that surrounds the Orientale impact feature. Just to the southeast i ... ryd ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
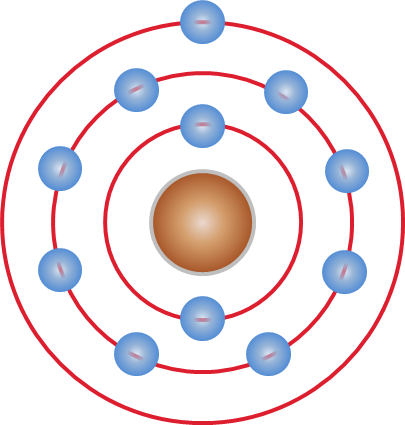
Janne Rydberg
Johannes (Janne) Robert Rydberg (; 8 November 1854 – 28 December 1919) was a Swedish physicist mainly known for devising the Rydberg formula, in 1888, which is used to describe the wavelengths of photons (of visible light and other electromagnetic radiation) emitted by changes in the energy level of an electron in a hydrogen atom. Biography Rydberg was born 8 November 1854 in Halmstad in southern Sweden, the only child of Sven Rydberg and Maria Anderson Rydberg. When he was 4 years old his father died, and the family was forced to live on a small income. In 1873 he graduated from Halmstads elementärläroverk, where he received high grades in maths and physics. Later that year he enrolled in Lund University, and two years later he was awarded his bachelor's degree. In 1879 he was awarded his Doctor of Philosophy with his dissertation "Konstruktioner af kägelsnitt i 3- och 4-punktskontakt". Rydberg began his career as an amanuensis in the institution. He became ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Rydberg Constant
In spectroscopy, the Rydberg constant, symbol R_\infty for heavy atoms or R_\text for hydrogen, named after the Swedish physicist Johannes Rydberg, is a physical constant relating to the electromagnetic spectra of an atom. The constant first arose as an empirical fitting parameter in the Rydberg formula for the hydrogen spectral series, but Niels Bohr later showed that its value could be calculated from more fundamental constants via his Bohr model. Before the redefinition of the SI base units in , R_\infty and the electron spin ''g''-factor were the most accurately measured physical constants. The constant is expressed for either hydrogen as R_\text, or at the limit of infinite nuclear mass as R_\infty. In either case, the constant is used to express the limiting value of the highest wavenumber (inverse wavelength) of any photon that can be emitted from an atom, or, alternatively, the wavenumber of the lowest-energy photon capable of ionizing an atom from its ground state. T ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Rydberg Correction
The term quantum defect refers to two concepts: energy loss in lasers and energy levels in alkali metal, alkali elements. Both deal with quantum systems where matter interacts with light. In laser science In laser science, the term "quantum defect" refers to the fact that the energy of a pump photon is generally higher than that of a ''signal photon'' (photon of the output radiation). The energy difference is lost to heat, which may carry away the excess entropy delivered by the multimode incoherent pump. The quantum defect of a laser can be defined as part of the energy of the pumping photon, which is lost (not turned into photons at the lasing wavelength) in the gain medium at the lasing. At given frequency \omega_ of pump and given frequency \omega_ of lasing, the quantum defect q = \hbar \omega_ - \hbar\omega_. Such quantum defect has dimension of energy; for the efficient operation, the temperature of the gain medium (measured in units of energy) should be small compared to th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Rydberg Formula
In atomic physics, the Rydberg formula calculates the wavelengths of a spectral line in many chemical elements. The formula was primarily presented as a generalization of the Balmer series for all atomic electron transitions of hydrogen. It was first empirically stated in 1888 by the Swedish physicist Johannes Rydberg, then theoretically by Niels Bohr in 1913, who used a primitive form of quantum mechanics. The formula directly generalizes the equations used to calculate the wavelengths of the hydrogen spectral series. History In 1880, Rydberg worked on a formula describing the relation between the wavelengths in spectral lines of alkali metals. He noticed that lines came in series and he found that he could simplify his calculations using the wavenumber (the number of waves occupying the unit length, equal to 1/''λ'', the inverse of the wavelength) as his unit of measurement. He plotted the wavenumbers (''n'') of successive lines in each series against consecutive integers w ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Rydberg Ionization Spectroscopy
Rydberg ionization spectroscopy is a spectroscopy technique in which multiple photons are absorbed by an atom causing the removal of an electron to form an ion. Resonance ionization spectroscopy The ionization threshold energy of atoms and small molecules are typically larger than the photon energies that are most easily available experimentally. However, it can be possible to span this ionization threshold energy if the photon energy is resonant with an intermediate electronically excited state. While it is often possible to observe the lower Rydberg levels in conventional spectroscopy of atoms and small molecules, Rydberg states are even more important in laser ionization experiments. Laser spectroscopic experiments often involve ionization through a photon energy resonance at an intermediate level, with an unbound final electron state and an ionic core. On resonance for phototransitions permitted by selection rules, the intensity of the laser in combination with the excited sta ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Rydberg State
The Rydberg states of an atom or molecule are electronically excited states with energies that follow the Rydberg formula as they converge on an ionic state with an ionization energy. Although the Rydberg formula was developed to describe atomic energy levels, it has been used to describe many other systems that have electronic structure roughly similar to atomic hydrogen. In general, at sufficiently high principal quantum numbers, an excited electron-ionic core system will have the general character of a hydrogenic system and the energy levels will follow the Rydberg formula. Rydberg states have energies converging on the energy of the ion. The ionization energy threshold is the energy required to completely liberate an electron from the ionic core of an atom or molecule. In practice, a Rydberg wave packet is created by a laser pulse on a hydrogenic atom and thus populates a superposition of Rydberg states. Modern investigations using pump-probe experiments show molecular pathways ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Heavy Rydberg System
A heavy Rydberg system consists of a weakly bound positive and negative ion orbiting their common centre of mass. Such systems share many properties with the conventional Rydberg atom and consequently are sometimes referred to as heavy Rydberg atoms. While such a system is a type of ionically bound molecule, it should not be confused with a molecular Rydberg state, which is simply a molecule with one or more highly excited electrons. The peculiar properties of the Rydberg atom come from the large charge separation and the resulting hydrogenic potential. The extremely large separation between the two components of a heavy Rydberg system results in an almost perfect ''1/r'' hydrogenic potential seen by each ion. The positive ion can be viewed as analogous to the nucleus of a hydrogen atom, with the negative ion playing the role of the electron. Species The most commonly studied system to date is the H^+/H^- system, consisting of a proton bound with a H^- ion. The H^+/H^- system wa ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Rydberg Atom
A Rydberg atom is an excited atom with one or more electrons that have a very high principal quantum number, ''n''. The higher the value of ''n'', the farther the electron is from the nucleus, on average. Rydberg atoms have a number of peculiar properties including an exaggerated response to electric and magnetic fields, long decay periods and electron wavefunctions that approximate, under some conditions, classical orbits of electrons about the nuclei. The core electrons shield the outer electron from the electric field of the nucleus such that, from a distance, the electric potential looks identical to that experienced by the electron in a hydrogen atom. In spite of its shortcomings, the Bohr model of the atom is useful in explaining these properties. Classically, an electron in a circular orbit of radius ''r'', about a hydrogen nucleus of charge +'' e'', obeys Newton's second law: : \mathbf=m\mathbf \Rightarrow = where ''k'' = 1/(4π ε0). Orbital momentum is quantiz ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Rydberg Matter
Rydberg matter is an exotic phase of matter formed by Rydberg atoms; it was predicted around 1980 by É. A. Manykin, M. I. Ozhovan and P. P. Poluéktov. It has been formed from various elements like caesium, potassium, hydrogen and nitrogen; studies have been conducted on theoretical possibilities like sodium, beryllium, magnesium and calcium. It has been suggested to be a material that diffuse interstellar bands may arise from. Circular Rydberg states, where the outermost electron is found in a planar circular orbit, are the most long-lived, with lifetimes of up to several hours, and are the most common. Physical Rydberg matter consists of usually hexagonal planar clusters; these cannot be very big because of the retardation effect caused by the finite velocity of the speed of light. Hence, they are not gases or plasmas; nor are they solids or liquids; they are most similar to dusty plasmas with small clusters in a gas. Though Rydberg matter can be studied in the laborator ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Rydberg Molecule
A Rydberg molecule is an electronically excited chemical species. Electronically excited molecular states are generally quite different in character from electronically excited atomic states. However, particularly for highly electronically excited molecular systems, the ionic core interaction with an excited electron can take on the general aspects of the interaction between the proton and the electron in the hydrogen atom. The spectroscopic assignment of these states follows the Rydberg formula, named after the Swedish physicist Johannes Rydberg, and they are called Rydberg states of molecules. Rydberg series are associated with partially removing an electron from the ionic core. Each Rydberg series of energies converges on an ionization energy threshold associated with a particular ionic core configuration. These quantized Rydberg energy levels can be associated with the quasiclassical Bohr atomic picture. The closer you get to the ionization threshold energy, the higher the prin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Rydberg Polaron
A Rydberg polaron is an exotic state of matter, created at low temperatures, in which a very large atom contains other ordinary atoms in the space between the nucleus and the electrons. For the formation of this atom, scientists had to combine two fields of atomic physics: Bose–Einstein condensates and Rydberg atoms. Rydberg atoms are formed by exciting a single atom into a high-energy state, in which the electron is very far from the nucleus. Bose–Einstein condensates are a state of matter that is produced at temperatures close to absolute zero. Polarons are induced by using a laser to excite Rydberg atoms contained as impurities in a Bose–Einstein condensate. In those Rydberg atoms, the average distance between the electron and its nucleus can be as large as several hundred nanometres, which is more than a thousand times the radius of a hydrogen atom. Under these circumstances, the distance between the nucleus and the electron of the excited Rydberg atoms is higher than the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Rydberg–Klein–Rees Method
The Rydberg–Klein–Rees method is a procedure used in the analysis of rotational-vibrational spectra of diatomic molecules to obtain a potential energy In physics, potential energy is the energy held by an object because of its position relative to other objects, stresses within itself, its electric charge, or other factors. Common types of potential energy include the gravitational potentia ... curve from the experimentally-known line positions. Atomic physics {{AMO-physics-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |