|
Li Gan
Li Gan is a neuroscientist and professor at Weill Cornell Medical College. She is known for her discovery of pathogenic tau protein acetylation in tauopathies and mechanisms of microglia dysfunction in neurodegeneration. Education and career Gan attended Peking University from 1986 to 1990 and earned a BS in Physiology. She then attended Yale School of Medicine, where she was advised by Leonard K. Kaczmarek and studied voltage-gated potassium channels in high frequency-firing neurons. Gan received her PhD in Cellular & Molecular Physiology in 1996. Gan conducted postdoctoral studies with Gerald Fischbach at Harvard Medical School and with Lennart Mucke at the Gladstone Institute of Neurological Disease. From 2000 to 2003, she worked at AGY Therapeutics Inc., a biotechnology company based in South San Francisco, CA. In 2003, Gan joined the Gladstone Institute as a staff research investigator and became an assistant adjunct professor in neurology at the University of Califor ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Induced Pluripotent Stem Cell
Induced pluripotent stem cells (also known as iPS cells or iPSCs) are a type of pluripotent stem cell that can be generated directly from a somatic cell. The iPSC technology was pioneered by Shinya Yamanaka's lab in Kyoto, Japan, who showed in 2006 that the introduction of four specific genes (named Myc, Oct3/4, Sox2 and Klf4), collectively known as Yamanaka factors, encoding transcription factors could convert somatic cells into pluripotent stem cells. He was awarded the 2012 Nobel Prize along with Sir John Gurdon "for the discovery that mature cells can be reprogrammed to become pluripotent." Pluripotent stem cells hold promise in the field of regenerative medicine. Because they can propagate indefinitely, as well as give rise to every other cell type in the body (such as neurons, heart, pancreatic, and liver cells), they represent a single source of cells that could be used to replace those lost to damage or disease. The most well-known type of pluripotent stem cell is the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Proteasome
Proteasomes are protein complexes which degrade unneeded or damaged proteins by proteolysis, a chemical reaction that breaks peptide bonds. Enzymes that help such reactions are called proteases. Proteasomes are part of a major mechanism by which cells regulate the concentration of particular proteins and degrade misfolded proteins. Proteins are tagged for degradation with a small protein called ubiquitin. The tagging reaction is catalyzed by enzymes called ubiquitin ligases. Once a protein is tagged with a single ubiquitin molecule, this is a signal to other ligases to attach additional ubiquitin molecules. The result is a ''polyubiquitin chain'' that is bound by the proteasome, allowing it to degrade the tagged protein. The degradation process yields peptides of about seven to eight amino acids long, which can then be further degraded into shorter amino acid sequences and used in synthesizing new proteins. Proteasomes are found inside all eukaryotes and archaea, and in so ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Amyloid
Amyloids are aggregates of proteins characterised by a Fibril, fibrillar morphology of 7–13 Nanometer, nm in diameter, a beta sheet (β-sheet) Secondary structure of proteins, secondary structure (known as cross-β) and ability to be Staining, stained by particular dyes, such as Congo red. In the human body, amyloids have been linked to the development of various diseases. Pathogenic amyloids form when previously healthy proteins lose their normal Protein structure, structure and physiology, physiological functions (Protein misfolding, misfolding) and form fibrous deposits in amyloid plaques around cells which can disrupt the healthy function of tissues and organs. Such amyloids have been associated with (but not necessarily as the cause of) more than 50 human diseases, known as amyloidosis, and may play a role in some neurodegenerative diseases. Some of these diseases are mainly sporadic and only a few cases are Genetic disorder, familial. Others are only Genetic disorder, fam ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cystatin C
Cystatin C or cystatin 3 (formerly gamma trace, post-gamma-globulin, or neuroendocrine basic polypeptide), a protein encoded by the CST3 gene, is mainly used as a biomarker of kidney function. Recently, it has been studied for its role in predicting new-onset or deteriorating cardiovascular disease. It also seems to play a role in brain disorders involving amyloid (a specific type of protein deposition), such as Alzheimer's disease. In humans, all Cell (biology), cells with a Cell nucleus, nucleus (cell core containing the DNA) produce cystatin C as a polypeptide, chain of 120 amino acids. It is found in virtually all tissues and body fluids. It is a potent enzyme inhibitor, inhibitor of lysosome, lysosomal proteinases (enzymes from a special organelle, subunit of the cell that break down proteins) and probably one of the most important extracellular inhibitors of cysteine proteases (it prevents the breakdown of proteins outside the cell by a specific type of protein degrading enzyme ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cathepsin B
Cathepsin B belongs to a family of lysosomal cysteine proteases known as the cysteine cathepsins and plays an important role in intracellular proteolysis. In humans, cathepsin B is encoded by the ''CTSB'' gene. Cathepsin B is upregulated in certain cancers, in pre-malignant lesions, and in various other pathological conditions. Structure Gene The ''CTSB'' gene is located at chromosome 8p22, consisting of 13 exons. The promoter of CTSB gene contains a GC-rich region including many SP1 sites, which is similar to housekeeping genes. At least five transcript variants encoding the same protein have been found for this gene. Protein Cathepsin B is synthesized on the rough endoplasmic reticulum as a preproenzyme of 339 amino acids with a signal peptide of 17 amino acids. Procathepsin B of 43/46 kDa is then transported to the Golgi apparatus, where cathepsin B is formed. Mature cathepsin B is composed of a heavy chain of 25-26 kDa and a light chain of 5kDa, which are linked by a dime ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Proteostasis
Proteostasis is the dynamic regulation of a balanced, functional proteome. The proteostasis network includes competing and integrated biological pathways within cells that control the biogenesis, folding, trafficking, and degradation of proteins present within and outside the cell. Loss of proteostasis is central to understanding the cause of diseases associated with excessive protein misfolding and degradation leading to loss-of-function phenotypes, as well as aggregation-associated degenerative disorders. Therapeutic restoration of proteostasis may treat or resolve these pathologies. Cellular proteostasis is key to ensuring successful development, healthy aging, resistance to environmental stresses, and to minimize homeostatic perturbations from pathogens such as viruses. Cellular mechanisms for maintaining proteostasis include regulated protein translation, chaperone assisted protein folding, and protein degradation pathways. Adjusting each of these mechanisms based on the need f ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
TREM2
Triggering receptor expressed on myeloid cells 2 (TREM2) is a protein that in humans is encoded by the ''TREM2'' gene. TREM2 is expressed on macrophages, immature monocyte-derived dendritic cells, osteoclasts, and microglia, which are immune cells in the central nervous system. In the liver, TREM2 is expressed by several cell types, including macrophages, that respond to injury. In the intestine, TREM2 is expressed by myeloid-derived dendritic cells and macrophage. TREM2 is overexpressed in many tumor types and has anti-inflammatory activities. It might therefore be a good therapeutic target. Gene The TREM2' gene lies on the sixth chromosome in humans, specifically in location 6p21.1. The gene has 5 coding exon regions. Alternative splicing of the ''TREM2'' mRNA transcript leads to different isoforms of the protein being produced upon translation. Specifically, ''TREM2'' mRNA has 3 different isoforms containing 3 consistent exons, and 2 that vary between the isoforms. ''TREM2'' ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
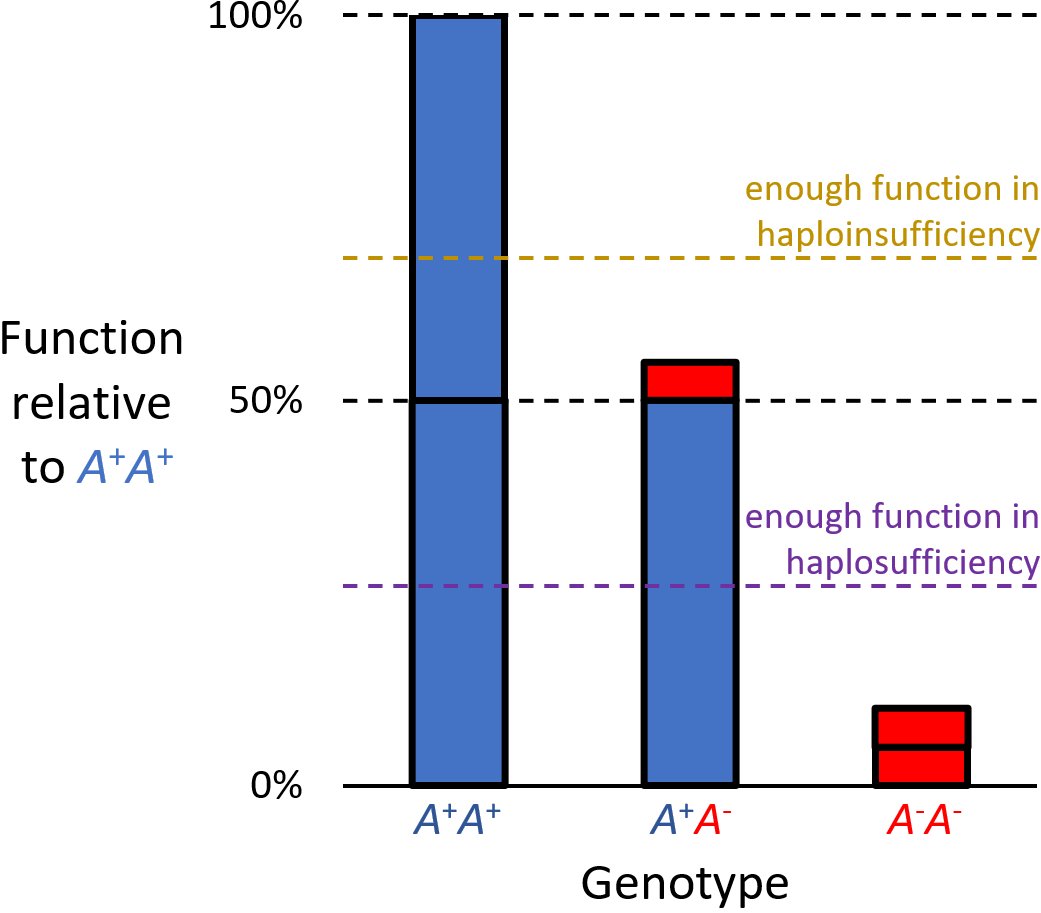
Haploinsufficiency
Haploinsufficiency in genetics describes a model of dominant gene action in diploid organisms, in which a single copy of the wild-type allele at a locus in heterozygous combination with a variant allele is insufficient to produce the wild-type phenotype. Haploinsufficiency may arise from a ''de novo'' or inherited loss-of-function mutation in the variant allele, such that it yields little or no gene product (often a protein). Although the other, standard allele still produces the standard amount of product, the total product is insufficient to produce the standard phenotype. This heterozygous genotype may result in a non- or sub-standard, deleterious, and (or) disease phenotype. Haploinsufficiency is the standard explanation for dominant deleterious alleles. In the alternative case of haplosufficiency, the loss-of-function allele behaves as above, but the single standard allele in the heterozygous genotype produces sufficient gene product to produce the same, standard phenotype ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Interleukin 1 Beta
Interleukin-1 beta (IL-1β) also known as leukocytic pyrogen, leukocytic endogenous mediator, mononuclear cell factor, lymphocyte activating factor and other names, is a cytokine protein that in humans is encoded by the ''IL1B'' gene."Catabolin" is the name given by Jeremy Saklatvala for IL-1 alpha. There are two genes for interleukin-1 (IL-1): IL-1 alpha and IL-1 beta (this gene). IL-1β precursor is cleaved by cytosolic caspase 1 (interleukin 1 beta convertase) to form mature IL-1β. Function The fever-producing property of human leukocytic pyrogen (interleukin 1) was purified by Dinarello in 1977 with a specific activity of 10–20 nanograms/kg. In 1979, Dinarello reported that purified human leukocytic pyrogen was the same molecule that was described by Igal Gery in 1972. He named it lymphocyte-activating factor (LAF) because it was a lymphocyte mitogen. It was not until 1984 that interleukin 1 was discovered to consist of two distinct proteins, now called interleukin-1 a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Resveratrol
Resveratrol (3,5,4′-trihydroxy-''trans''-stilbene) is a stilbenoid, a type of natural phenol, and a phytoalexin produced by several plants in response to injury or when the plant is under attack by pathogens, such as bacteria or fungi. Sources of resveratrol in food include the skin of grapes, blueberries, raspberries, mulberries, and peanuts. Although commonly used as a dietary supplement and studied in laboratory models of human diseases, there is no high-quality evidence that resveratrol improves lifespan or has a substantial effect on any human disease. Research Resveratrol has been studied for its potential therapeutic use, with little evidence of anti-disease effects or health benefits in humans. Cardiovascular disease There is no evidence of benefit from resveratrol in people who already have heart disease. A 2018 meta-analysis found no effect on systolic or diastolic blood pressure; a sub-analysis revealed a 2 mmHg decrease in systolic pressure only from res ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sirtuin 1
Sirtuin 1, also known as NAD-dependent deacetylase sirtuin-1, is a protein that in humans is encoded by the SIRT1 gene. SIRT1 stands for sirtuin (silent mating type information regulation 2 homolog) 1 (''S. cerevisiae''), referring to the fact that its sirtuin homolog (biological equivalent across species) in yeast ''(Saccharomyces cerevisiae)'' is Sir2. SIRT1 is an enzyme located primarily in the cell nucleus that deacetylates transcription factors that contribute to cellular regulation (reaction to stressors, longevity). Function Sirtuin 1 is a member of the sirtuin family of proteins, homologs of the Sir2 gene in ''S. cerevisiae''. Members of the sirtuin family are characterized by a sirtuin core domain and grouped into four classes. The functions of human sirtuins have not yet been determined; however, yeast sirtuin proteins are known to regulate epigenetic gene silencing and suppress recombination of rDNA. Studies suggest that the human sirtuins may function as intracel ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
.jpg)