|
Larry Overman
Larry E. Overman is Distinguished Professor of Chemistry at the University of California, Irvine. He was born in Chicago in 1943. Overman obtained a B.A. degree from Earlham College in 1965, and he completed his Ph.D. in chemistry from the University of Wisconsin–Madison in 1969, under Howard Whitlock Jr. Professor Overman is a member of the United States National Academy of Sciences and the American Academy of Arts and Sciences. He was the recipient of the Arthur C. Cope Award in 2003, and he was awarded the Tetrahedron Prize for Creativity in Organic Chemistry for 2008. Overman's research is focused on the development of new chemical reactions, particularly transition metal catalyzed reactions, and the application of those reactions toward the synthesis of natural products. Overman is most known for the Overman rearrangement, a Claisen rearrangement of allylic alcohols to give allylic trichloroacetamides. Career Overman's Ph.D. focused on the mechanism of rearrangemen ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
University Of California, Irvine
The University of California, Irvine (UCI or UC Irvine) is a public land-grant research university in Irvine, California. One of the ten campuses of the University of California system, UCI offers 87 undergraduate degrees and 129 graduate and professional degrees, and roughly 30,000 undergraduates and 6,000 graduate students are enrolled at UCI as of Fall 2019. The university is classified among " R1: Doctoral Universities – Very high research activity", and had $436.6 million in research and development expenditures in 2018. UCI became a member of the Association of American Universities in 1996. The university was rated as one of the "Public Ivies” in 1985 and 2001 surveys comparing publicly funded universities the authors claimed provide an education comparable to the Ivy League. The university also administers the UC Irvine Medical Center, a large teaching hospital in Orange, and its affiliated health sciences system; the University of California, Irvine, Arboretum; and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Strychnine
Strychnine (, , US chiefly ) is a highly toxic, colorless, bitter, crystalline alkaloid used as a pesticide, particularly for killing small vertebrates such as birds and rodents. Strychnine, when inhaled, swallowed, or absorbed through the eyes or mouth, causes poisoning which results in muscular convulsions and eventually death through asphyxia. While it is no longer used medicinally, it was used historically in small doses to strengthen muscle contractions, such as a heart and bowel stimulant and performance-enhancing drug. The most common source is from the seeds of the ''Strychnos nux-vomica'' tree. Biosynthesis Strychnine is a terpene indole alkaloid belonging to the ''Strychnos'' family of '' Corynanthe'' alkaloids, and it is derived from tryptamine and secologanin. The biosynthesis of strychine was solved in 2022. The enzyme, strictosidine synthase, catalyzes the condensation of tryptamine and secologanin, followed by a Pictet-Spengler reaction to form strictosidine ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gephyrotoxin
Gephyrotoxin is a naturally occurring product that stems from the Colombian tropical frog '' Dendrobates histrionicus''. It is a member of the class of compounds known as histrionicotoxins. This alkaloid skin secretion was first isolated from the tropical frog in 1977 by Daly and his fellow workers. Biological uses This compound is a relatively non-toxic chemical. At first it showed activity as a slight muscarinic antagonist, but with recent studies it has showed other interesting neurological activities. Due to these new activities, many laboratories want to conduct future research on it. Due to this demand and the scarcity and lack of abundance of the tree frog the synthesis of this product is of much interest. Synthesis The first total synthesis of gephyrotoxin was performed by Kishi and his co-workers, here they prepped an intermediate from L-pyroglutamic acid Pyroglutamic acid (also known as PCA, 5-oxoproline, pidolic acid) is a ubiquitous but little studied natura ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Samuel J
Samuel ''Šəmūʾēl'', Tiberian: ''Šămūʾēl''; ar, شموئيل or صموئيل '; el, Σαμουήλ ''Samouḗl''; la, Samūēl is a figure who, in the narratives of the Hebrew Bible, plays a key role in the transition from the biblical judges to the United Kingdom of Israel under Saul, and again in the monarchy's transition from Saul to David. He is venerated as a prophet in Judaism, Christianity, and Islam. In addition to his role in the Hebrew scriptures, Samuel is mentioned in Jewish rabbinical literature, in the Christian New Testament, and in the second chapter of the Quran (although Islamic texts do not mention him by name). He is also treated in the fifth through seventh books of '' Antiquities of the Jews'', written by the Jewish scholar Josephus in the first century. He is first called "the Seer" in 1 Samuel 9:9. Biblical account Family Samuel's mother was Hannah and his father was Elkanah. Elkanah lived at Ramathaim in the district of Zuph. His geneal ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pumiliotoxin
Pumiliotoxins (PTXs), are one of several toxins found in the skin of poison dart frogs. The frog species, P. bibronii also produces PTXs to deter predators. Closely related, though more toxic, are allopumiliotoxins, (aPTXs). Other toxins found in the skin of poison frogs include decahydroquinolines (DHQs), izidines, coccinellines, and spiropyrrolizidine alkaloids. Pumiliotoxins are very poisonous in high concentrations. Pumiliotoxins are much weaker than batrachotoxins, ranging between 100 and 1000 times less poisonous. There are three different types of this toxin: A, B and C, of which toxins A and B are more toxic than C. Pumiliotoxins interfere with muscle contraction by affecting calcium channels, causing partial paralysis, difficulty moving, hyperactivity, or death. The median lethal dose of pumiliotoxins A and B is 50 µg / mouse, 20 µg / mouse respectively, while the amount of pumiliotoxin is 200 µg / frog.http://wiredspace.wits.ac.za/bitstream/handle/1053 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Heck Reaction
The Heck reaction (also called the Mizoroki–Heck reaction) is the chemical reaction of an unsaturated halide (or triflate) with an alkene in the presence of a base and a palladium catalyst (or palladium nanomaterial-based catalyst) to form a substituted alkene. It is named after Tsutomu Mizoroki and Richard F. Heck. Heck was awarded the 2010 Nobel Prize in Chemistry, which he shared with Ei-ichi Negishi and Akira Suzuki, for the discovery and development of this reaction. This reaction was the first example of a carbon-carbon bond-forming reaction that followed a Pd(0)/Pd(II) catalytic cycle, the same catalytic cycle that is seen in other Pd(0)-catalyzed cross-coupling reactions. The Heck reaction is a way to substitute alkenes. History The original reaction by Tsutomu Mizoroki (1971) describes the coupling between iodobenzene and styrene in methanol to form stilbene at 120 °C (autoclave) with potassium acetate base and palladium chloride catalysis. This work was an ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
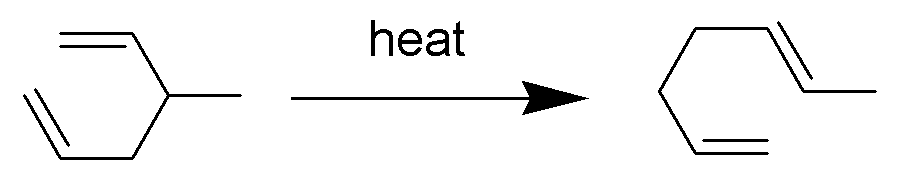
Cope Rearrangement
The Cope rearrangement is an extensively studied organic reaction involving the ,3sigmatropic rearrangement of 1,5-dienes. It was developed by Arthur C. Cope and Elizabeth Hardy. For example, 3-methyl-hexa-1,5-diene heated to 300 °C yields hepta-1,5-diene. The Cope rearrangement causes the fluxional states of the molecules in the bullvalene family. Mechanism The Cope rearrangement is the prototypical example of a concerted sigmatropic rearrangement. It is classified as a ,3sigmatropic rearrangement with the Woodward–Hoffmann symbol π2s+σ2s+π2s.html" ;"title="sub>π2s+σ2s+π2s">sub>π2s+σ2s+π2sand is therefore thermally allowed. It is sometimes useful to think of it as going through a transition state energetically and structurally equivalent to a diradical, although the diradical is not usually a true intermediate (potential energy minimum). The chair transition state illustrated here is preferred in open-chain systems (as shown by the Doering-Roth experiments ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Journal Of The American Chemical Society
The ''Journal of the American Chemical Society'' is a weekly peer-reviewed scientific journal that was established in 1879 by the American Chemical Society. The journal has absorbed two other publications in its history, the ''Journal of Analytical and Applied Chemistry'' (July 1893) and the ''American Chemical Journal'' (January 1914). It covers all fields of chemistry. Since 2021, the editor-in-chief is Erick M. Carreira (ETH Zurich). In 2014, the journal moved to a hybrid open access publishing model. Abstracting and indexing The journal is abstracted and indexed in Chemical Abstracts Service, Scopus, EBSCO databases, ProQuest databases, Index Medicus/MEDLINE/PubMed, and the Science Citation Index Expanded. According to the ''Journal Citation Reports'', the journal has a 2021 impact factor of 16.383. Editors-in-chief The following people are or have been editor-in-chief: * 1879–1880 – Hermann Endemann * 1880–1881 – Gideon E. Moore * 1881–1882 – Hermann Endemann ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cyclodextrin
Cyclodextrins are a family of cyclic oligosaccharides, consisting of a macrocyclic ring of glucose subunits joined by α-1,4 glycosidic bonds. Cyclodextrins are produced from starch by enzymatic conversion. They are used in food, pharmaceutical, drug delivery, and chemical industries, as well as agriculture and environmental engineering. Cyclodextrins are composed of 5 or more α-D-glucopyranoside units linked 1->4, as in amylose (a fragment of starch). Typical cyclodextrins contain a number of glucose monomers ranging from six to eight units in a ring, creating a cone shape: * α (alpha)-cyclodextrin: 6 glucose subunits * β (beta)-cyclodextrin: 7 glucose subunits * γ (gamma)-cyclodextrin: 8 glucose subunits The largest well-characterized cyclodextrin contains 32 1,4-anhydroglucopyranoside units. Poorly-characterized mixtures, containing at least 150-membered cyclic oligosaccharides are also known. Applications Drug delivery Cyclodextrins are ingredients in more than 30 d ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ronald Breslow
Ronald Charles David Breslow (March 14, 1931 – October 25, 2017) was an American chemist from Rahway, New Jersey. He was University Professor at Columbia University, where he was based in the Department of Chemistry and affiliated with the Departments of Biological Sciences and Pharmacology; he had also been on the faculty of its Department of Chemical Engineering. He had taught at Columbia since 1956 and was a former chair of the university's chemistry department. Life and career Breslow was born in Rahway, New Jersey, the son of Gladys (Fellows) and Alexander E. Breslow. He was interested in the design and synthesis of new molecules with interesting properties, and the study of these properties. Examples include the cyclopropenyl cation, the simplest aromatic system and the first aromatic compound prepared with other than six electrons in a ring. His seminal contributions include the correct site of reactivity of thiamin diphosphate in enzymes that promote the decarboxy ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |