|
Laboratory Information System
A laboratory information management system (LIMS), sometimes referred to as a laboratory information system (LIS) or laboratory management system (LMS), is a software-based solution with features that support a modern laboratory's operations. Key features include—but are not limited to—workflow and data tracking support, flexible architecture, and data exchange interfaces, which fully "support its use in regulated environments". The features and uses of a LIMS have evolved over the years from simple sample tracking to an enterprise resource planning tool that manages multiple aspects of laboratory informatics. There is no useful definition of the term "LIMS" as it is used to encompass a number of different laboratory informatics components. The spread and depth of these components is highly dependent on the LIMS implementation itself. All LIMSs have a workflow component and some summary data management facilities but beyond that there are significant differences in functio ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sequencing
In genetics and biochemistry, sequencing means to determine the primary structure (sometimes incorrectly called the primary sequence) of an unbranched biopolymer. Sequencing results in a symbolic linear depiction known as a sequence which succinctly summarizes much of the atomic-level structure of the sequenced molecule. DNA sequencing DNA sequencing is the process of determining the nucleotide order of a given DNA fragment. So far, most DNA sequencing has been performed using the chain termination method developed by Frederick Sanger. This technique uses sequence-specific termination of a DNA synthesis reaction using modified nucleotide substrates. However, new sequencing technologies such as pyrosequencing are gaining an increasing share of the sequencing market. More genome data are now being produced by pyrosequencing than Sanger DNA sequencing. Pyrosequencing has enabled rapid genome sequencing. Bacterial genomes can be sequenced in a single run with several times cover ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electronic Signature
An electronic signature, or e-signature, is data that is logically associated with other data and which is used by the signatory to sign the associated data. This type of signature has the same legal standing as a handwritten signature as long as it adheres to the requirements of the specific regulation under which it was created (e.g., eIDAS in the European Union, NIST-DSS in the USA or ZertES in Switzerland). Electronic signatures are a legal concept distinct from digital signatures, a cryptographic mechanism often used to implement electronic signatures. While an electronic signature can be as simple as a name entered in an electronic document, digital signatures are increasingly used in e-commerce and in regulatory filings to implement electronic signatures in a cryptographically protected way. Standardization agencies like NIST or ETSI provide standards for their implementation (e.g., NIST-DSS, XAdES or PAdES). The concept itself is not new, with common law jurisdictions h ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Food And Drug Administration
The United States Food and Drug Administration (FDA or US FDA) is a List of United States federal agencies, federal agency of the United States Department of Health and Human Services, Department of Health and Human Services. The FDA is responsible for protecting and promoting public health through the control and supervision of food safety, tobacco products, caffeine products, dietary supplements, Prescription drug, prescription and Over-the-counter drug, over-the-counter pharmaceutical drugs (medications), vaccines, biopharmaceuticals, blood transfusions, medical devices, electromagnetic radiation emitting devices (ERED), cosmetics, Animal feed, animal foods & feed and Veterinary medicine, veterinary products. The FDA's primary focus is enforcement of the Federal Food, Drug, and Cosmetic Act (FD&C), but the agency also enforces other laws, notably Section 361 of the Public Health Service Act, as well as associated regulations. Much of this regulatory-enforcement work is not d ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Good Laboratory Practice
In the experimental (non-clinical) research arena, good laboratory practice or GLP is a quality system of management controls for research laboratories and organizations to ensure the uniformity, consistency, reliability, reproducibility, quality, and integrity of products in development for human or animal health (including pharmaceuticals) through non-clinical safety tests; from physio-chemical properties through acute to chronic toxicity tests. GLP was first introduced in New Zealand and Denmark in 1972, and later in the US in 1978 in response to the Industrial BioTest Labs scandal. It was followed a few years later by the Organization for Economic Co-operation and Development (OECD) Principles of GLP in 1992; the OECD has since helped promulgate GLP to many countries. GLP applies to non-clinical studies conducted for the assessment of the safety or efficacy of products in development (including pharmaceuticals) for people, animals, and the environment. GLP, a data and opera ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
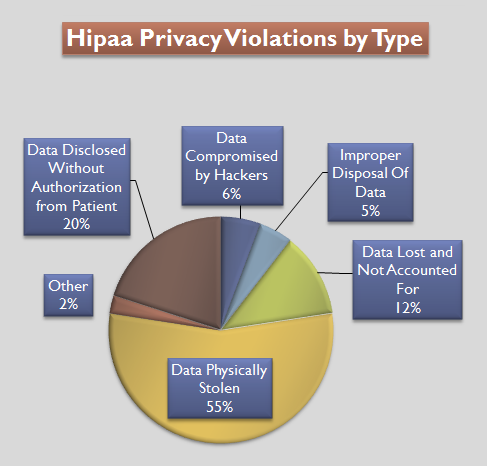
Health Insurance Portability And Accountability Act
The Health Insurance Portability and Accountability Act of 1996 (HIPAA or the Kennedy– Kassebaum Act) is a United States Act of Congress enacted by the 104th United States Congress and signed into law by President Bill Clinton on August 21, 1996. It modernized the flow of healthcare information, stipulates how personally identifiable information maintained by the healthcare and healthcare insurance industries should be protected from fraud and theft, and addressed some limitations on healthcare insurance coverage. It generally prohibits healthcare providers and healthcare businesses, called ''covered entities'', from disclosing protected information to anyone other than a patient and the patient's authorized representatives without their consent. With limited exceptions, it does not restrict patients from receiving information about themselves. It does not prohibit patients from voluntarily sharing their health information however they choose, nor does it require confidential ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Clinical Laboratory Improvement Amendments
The Clinical Laboratory Improvement Amendments (CLIA) of 1988 are United States federal regulatory standards that apply to all clinical laboratory testing performed on humans in the United States, except clinical trials and basic research. CLIA Program In accord with the CLIA, the CLIA Program sets standards and issues certificates for clinical laboratory testing. CLIA defines a clinical laboratory as any facility which performs laboratory testing on specimens derived from humans for the purpose of providing information for: * diagnosis, prevention, or treatment of disease or impairment * health assessments An objective of the CLIA is to ensure the accuracy, reliability and timeliness of test results regardless of where the test was performed. Most Laboratory Developed Tests have been regulated under this program. In 2014 the FDA started a public discussion about regulating some LDTs. Per CLIA, each specific laboratory system, assay, examination is graded for level of com ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phenotype
In genetics, the phenotype () is the set of observable characteristics or traits of an organism. The term covers the organism's morphology or physical form and structure, its developmental processes, its biochemical and physiological properties, its behavior, and the products of behavior. An organism's phenotype results from two basic factors: the expression of an organism's genetic code, or its genotype, and the influence of environmental factors. Both factors may interact, further affecting phenotype. When two or more clearly different phenotypes exist in the same population of a species, the species is called polymorphic. A well-documented example of polymorphism is Labrador Retriever coloring; while the coat color depends on many genes, it is clearly seen in the environment as yellow, black, and brown. Richard Dawkins in 1978 and then again in his 1982 book ''The Extended Phenotype'' suggested that one can regard bird nests and other built structures such as cad ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Barcode
A barcode or bar code is a method of representing data in a visual, machine-readable form. Initially, barcodes represented data by varying the widths, spacings and sizes of parallel lines. These barcodes, now commonly referred to as linear or one-dimensional (1D), can be scanned by special optical scanners, called barcode readers, of which there are several types. Later, two-dimensional (2D) variants were developed, using rectangles, dots, hexagons and other patterns, called ''matrix codes'' or ''2D barcodes'', although they do not use bars as such. 2D barcodes can be read using purpose-built 2D optical scanners, which exist in a few different forms. 2D barcodes can also be read by a digital camera connected to a microcomputer running software that takes a photographic image of the barcode and analyzes the image to deconstruct and decode the 2D barcode. A mobile device with an inbuilt camera, such as smartphone, can function as the latter type of 2D barcode reader using special ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Accession Number (bioinformatics)
An accession number, in bioinformatics, is a unique identifier given to a DNA or protein sequence record to allow for tracking of different versions of that sequence record and the associated sequence over time in a single data repository. Because of its relative stability, accession numbers can be utilized as foreign key A foreign key is a set of attributes in a table that refers to the primary key of another table. The foreign key links these two tables. Another way to put it: In the context of relational databases, a foreign key is a set of attributes subject to ...s for referring to a sequence object, but not necessarily to a unique sequence. All sequence information repositories implement the concept of "accession number" but might do so with subtle variations. LRG Locus Reference Genomic (LRG) records have unique accession numbers starting with LRG_ followed by a number. They are recommended in thHuman Genome Variation Society Nomenclature guidelinesas stable genomic ref ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lab Test Label Code39 And Qr
Lab most often refers to: * Laboratory, a facility to conduct scientific research Lab or LAB may also refer to: Places * Láb, a village near Bratislava in western Slovakia * Lab (river), in north-eastern Kosovo People * ISO 639 code for the ancient Minoan language of Crete Music * LAB Records, a British independent record label * LAB (band), a Finnish band * L.A.B., a New Zealand reggae band Transportation * Linhas Aéreas Brasileiras, a defunct Brazilian airline * Lloyd Aéreo Boliviano, a defunct Bolivian airline * League of American Bicyclists Science and technology * Linear alkylbenzene * Lithosphere-Asthenosphere boundary, between layers of the Earth * Lactic acid bacteria * Lab color space * Lyman-alpha blob, in interstellar space * Linear alkylbenzene sulfonate Groups * Langile Abertzaleen Batzordeak, a Basque trade union * Lockerz advisory board * Los Angeles Baptist High School, US Other * Labrador Retriever, a dog breed * Legs, abs, and butt fitness workout ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
.jpg)