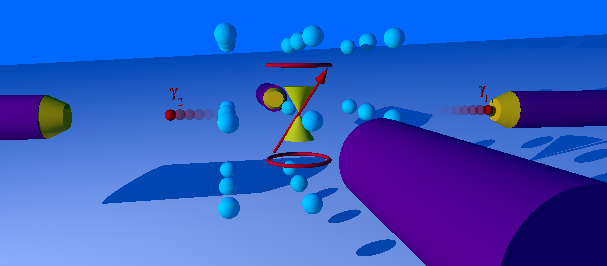|
Local Structure
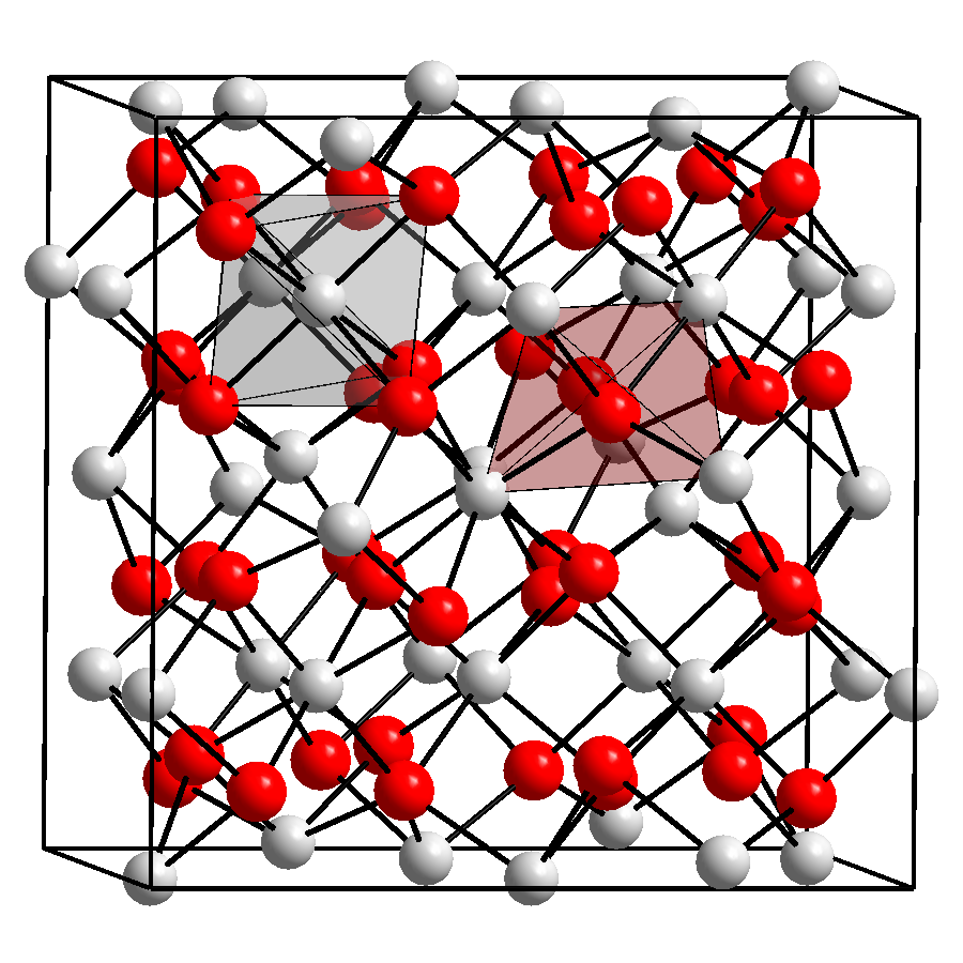
The local structure is a term in nuclear spectroscopy that refers to the structure of the nearest neighbours around an atom in crystals and molecules. E.g. in crystals the atoms order in a regular fashion on wide ranges to form even gigantic highly ordered crystals ( Naica Mine). However, in reality, crystals are never perfect and have impurities or defects, which means that a foreign atom resides on a lattice site or in between lattice sites (interstitials). These small defects and impurities cannot be seen by methods such as X-ray diffraction or neutron diffraction, because these methods average in their nature of measurement over a large number of atoms and thus are insensitive to effects in local structure. Methods in nuclear spectroscopy use specific nuclei as probe. The nucleus of an atom is about 10,000 to 150,000 times smaller than the atom itself. It experiences the electric fields created by the atom's electrons that surround the nucleus. In addition, the electric fields ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hyperfine Structure
In atomic physics, hyperfine structure is defined by small shifts in otherwise degenerate energy levels and the resulting splittings in those energy levels of atoms, molecules, and ions, due to electromagnetic multipole interaction between the nucleus and electron clouds. In atoms, hyperfine structure arises from the energy of the nuclear magnetic dipole moment interacting with the magnetic field generated by the electrons and the energy of the nuclear electric quadrupole moment in the electric field gradient due to the distribution of charge within the atom. Molecular hyperfine structure is generally dominated by these two effects, but also includes the energy associated with the interaction between the magnetic moments associated with different magnetic nuclei in a molecule, as well as between the nuclear magnetic moments and the magnetic field generated by the rotation of the molecule. Hyperfine structure contrasts with '' fine structure'', which results from the interact ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Atomic Physics
Atomic physics is the field of physics that studies atoms as an isolated system of electrons and an atomic nucleus. Atomic physics typically refers to the study of atomic structure and the interaction between atoms. It is primarily concerned with the way in which electrons are arranged around the nucleus and the processes by which these arrangements change. This comprises ions, neutral atoms and, unless otherwise stated, it can be assumed that the term ''atom'' includes ions. The term ''atomic physics'' can be associated with nuclear power and nuclear weapons, due to the synonymous use of ''atomic'' and ''nuclear'' in standard English. Physicists distinguish between atomic physics—which deals with the atom as a system consisting of a nucleus and electrons—and nuclear physics, which studies nuclear reactions and special properties of atomic nuclei. As with many scientific fields, strict delineation can be highly contrived and atomic physics is often considered in the wider ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electrostatics
Electrostatics is a branch of physics that studies electric charges at rest (static electricity). Since classical times, it has been known that some materials, such as amber, attract lightweight particles after rubbing. The Greek word for amber, (), was thus the source of the word 'electricity'. Electrostatic phenomena arise from the forces that electric charges exert on each other. Such forces are described by Coulomb's law. Even though electrostatically induced forces seem to be rather weak, some electrostatic forces are relatively large. The force between an electron and a proton, which together make up a hydrogen atom, is about 36 orders of magnitude stronger than the gravitational force acting between them. There are many examples of electrostatic phenomena, from those as simple as the attraction of plastic wrap to one's hand after it is removed from a package, to the apparently spontaneous explosion of grain silos, the damage of electronic components during manufa ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Giant Magnetoresistance
Giant magnetoresistance (GMR) is a quantum mechanical magnetoresistance effect observed in multilayers composed of alternating ferromagnetic and non-magnetic conductive layers. The 2007 Nobel Prize in Physics was awarded to Albert Fert and Peter Grünberg for the discovery of GMR. The effect is observed as a significant change in the electrical resistance depending on whether the magnetization of adjacent ferromagnetic layers are in a parallel or an antiparallel alignment. The overall resistance is relatively low for parallel alignment and relatively high for antiparallel alignment. The magnetization direction can be controlled, for example, by applying an external magnetic field. The effect is based on the dependence of electron scattering on spin orientation. The main application of GMR is in magnetic field sensors, which are used to read data in hard disk drives, biosensors, microelectromechanical systems (MEMS) and other devices. GMR multilayer structures are also used in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Perturbed Angular Correlation
The perturbed γ-γ angular correlation, PAC for short or PAC-Spectroscopy, is a method of nuclear solid-state physics with which magnetic and electric fields in crystal structures can be measured. In doing so, electrical field gradients and the Larmor frequency in magnetic fields as well as dynamic effects are determined. With this very sensitive method, which requires only about 10-1000 billion atoms of a radioactive isotope per measurement, material properties in the local structure, phase transitions, magnetism and diffusion can be investigated. The PAC method is related to nuclear magnetic resonance and the Mössbauer effect, but shows no signal attenuation at very high temperatures. Today only the time-differential perturbed angular correlation (TDPAC) is used. History and development PAC goes back to a theoretical work by Donald R. Hamilton from 1940. The first successful experiment was carried out by Brady and Deutsch in 1947. Essentially spin and parity of nuclear sp ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mössbauer Spectroscopy
Mössbauer spectroscopy is a spectroscopic technique based on the Mössbauer effect. This effect, discovered by Rudolf Mössbauer (sometimes written "Moessbauer", German: "Mößbauer") in 1958, consists of the nearly recoil-free emission and absorption of nuclear gamma rays in solids. The consequent nuclear spectroscopy method is exquisitely sensitive to small changes in the chemical environment of certain nuclei. Typically, three types of nuclear interactions may be observed: the isomer shift due to differences in nearby electron densities (also called the chemical shift in older literature), quadrupole splitting due to atomic-scale electric field gradients; and magnetic Zeeman splitting due to non-nuclear magnetic fields. Due to the high energy and extremely narrow line widths of nuclear gamma rays, Mössbauer spectroscopy is a highly sensitive technique in terms of energy (and hence frequency) resolution, capable of detecting changes of just a few parts in 1011. It is a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nuclear Magnetic Resonance
Nuclear magnetic resonance (NMR) is a physical phenomenon in which nuclei in a strong constant magnetic field are perturbed by a weak oscillating magnetic field (in the near field) and respond by producing an electromagnetic signal with a frequency characteristic of the magnetic field at the nucleus. This process occurs near resonance, when the oscillation frequency matches the intrinsic frequency of the nuclei, which depends on the strength of the static magnetic field, the chemical environment, and the magnetic properties of the isotope involved; in practical applications with static magnetic fields up to ca. 20 tesla, the frequency is similar to VHF and UHF television broadcasts (60–1000 MHz). NMR results from specific magnetic properties of certain atomic nuclei. Nuclear magnetic resonance spectroscopy is widely used to determine the structure of organic molecules in solution and study molecular physics and crystals as well as non-crystalline materials. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electric Field Gradient
In atomic, molecular, and solid-state physics, the electric field gradient (EFG) measures the rate of change of the electric field at an atomic nucleus generated by the electronic charge distribution and the other nuclei. The EFG couples with the nuclear electric quadrupole moment of quadrupolar nuclei (those with spin quantum number greater than one-half) to generate an effect which can be measured using several spectroscopic methods, such as nuclear magnetic resonance (NMR), microwave spectroscopy, electron paramagnetic resonance (EPR, ESR), nuclear quadrupole resonance (NQR), Mössbauer spectroscopy or perturbed angular correlation (PAC). The EFG is non-zero only if the charges surrounding the nucleus violate cubic symmetry and therefore generate an inhomogeneous electric field at the position of the nucleus. EFGs are highly sensitive to the electronic density in the immediate vicinity of a nucleus. This is because the EFG operator scales as ''r''−3, where ''r'' i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nuclear Spectroscopy
Nuclear spectroscopy is a superordinate concept of methods that uses properties of a nucleus to probe material properties. By emission or absorption of radiation from the nucleus information of the local structure is obtained, as an interaction of an atom with its closest neighbours. Or a radiation spectrum of the nucleus is detected. Most methods base on hyperfine interactions, which are the interaction of the nucleus with its interaction of its atom's electrons and their interaction with the nearest neighbor atoms as well as external fields. Nuclear spectroscopy is mainly applied to solids and liquids, rarely in gases. Its methods are important tools in condensed matter physics and solid state chemistry. Methods In nuclear physics these methods are used to study properties of the nucleus itself. Methods for studies of the nucleus: * Gamma spectroscopy * Hypernuclear spectroscopy Methods for condensed matter studies: * Nuclear magnetic resonance (NMR) * Mössbauer spect ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Atom
Every atom is composed of a nucleus and one or more electrons bound to the nucleus. The nucleus is made of one or more protons and a number of neutrons. Only the most common variety of hydrogen has no neutrons. Every solid, liquid, gas, and plasma is composed of neutral or ionized atoms. Atoms are extremely small, typically around 100 picometers across. They are so small that accurately predicting their behavior using classical physics, as if they were tennis balls for example, is not possible due to quantum effects. More than 99.94% of an atom's mass is in the nucleus. The protons have a positive electric charge, the electrons have a negative electric charge, and the neutrons have no electric charge. If the number of protons and electrons are equal, then the atom is electrically neutral. If an atom has more or fewer electrons than protons, then it has an overall negative or positive charge, respectively – such atoms are called ions. The electrons of an at ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Atomic Nucleus
The atomic nucleus is the small, dense region consisting of protons and neutrons at the center of an atom, discovered in 1911 by Ernest Rutherford based on the 1909 Geiger–Marsden experiments, Geiger–Marsden gold foil experiment. After the discovery of the neutron in 1932, models for a nucleus composed of protons and neutrons were quickly developed by Dmitri Ivanenko and Werner Heisenberg. An atom is composed of a positively charged nucleus, with a cloud of negatively charged electrons surrounding it, bound together by electrostatic force. Almost all of the mass of an atom is located in the nucleus, with a very small contribution from the electron cloud. Protons and neutrons are bound together to form a nucleus by the nuclear force. The diameter of the nucleus is in the range of () for hydrogen (the diameter of a single proton) to about for uranium. These dimensions are much smaller than the diameter of the atom itself (nucleus + electron cloud), by a factor of about 2 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |