|
Lipoglycopeptide
Lipoglycopeptides are a class of antibiotic that have lipophilic side-chains linked to glycopeptides. The class includes oritavancin, telavancin and dalbavancin. In September 2009 the US Food and Drug Administration (FDA) approved telavancin (Vibativ) for complicated skin and skin structure infections (cSSSI) On May 23, 2014, the FDA approved dalbavancin (Dalvance), an injectable drug, administered intravenously in two doses one week apart. On August 6, 2014, the FDA approved oritavancin (Orbactiv), an injectable drug administered as a single dose to comprise a full course of therapy. Telavancin is the most potent of the three against ''Clostridium spp.'' Approvals and clinical trials Telavancin (once daily injection) has completed 4 phase III trials. and gained Food and Drug Administration, US FDA approval in September 2009 for complicated skin and skin structure infections (cSSSI). Oritavancin (once daily injection) has completed phase II and phase III trials. Oritavancin wa ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dalbavancin
Dalbavancin, sold under the brand names Dalvance in the US and Xydalba in the EU among others, is a second-generation lipoglycopeptide antibiotic medication. It belongs to the same class as vancomycin, the most widely used and one of the treatments available to people infected with methicillin-resistant ''Staphylococcus aureus'' (MRSA). Dalbavancin is a semisynthetic lipoglycopeptide that was designed to improve upon the natural glycopeptides vancomycin and teicoplanin. It is derived from a complex of glycopeptide antibiotics, referred to as A-40926, that is produced by a new strain of Actinomadura. Dalbavancin has been referred to in the scientific literature by a series of names: MDL-63397, A-!-1, BI-397, VER-001. These different labels reflected where the research had been carried out: MDL representing Merrell-Dow-Lepetit, where the initial complex was discovered; BI referring to BioSearch Italia where Dalbavancin itself was first synthesized; VER referring to Versicor (wh ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dalbavancin
Dalbavancin, sold under the brand names Dalvance in the US and Xydalba in the EU among others, is a second-generation lipoglycopeptide antibiotic medication. It belongs to the same class as vancomycin, the most widely used and one of the treatments available to people infected with methicillin-resistant ''Staphylococcus aureus'' (MRSA). Dalbavancin is a semisynthetic lipoglycopeptide that was designed to improve upon the natural glycopeptides vancomycin and teicoplanin. It is derived from a complex of glycopeptide antibiotics, referred to as A-40926, that is produced by a new strain of Actinomadura. Dalbavancin has been referred to in the scientific literature by a series of names: MDL-63397, A-!-1, BI-397, VER-001. These different labels reflected where the research had been carried out: MDL representing Merrell-Dow-Lepetit, where the initial complex was discovered; BI referring to BioSearch Italia where Dalbavancin itself was first synthesized; VER referring to Versicor (wh ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Telavancin
Telavancin (trade name Vibativ) is a bactericidal lipoglycopeptide for use in MRSA or other Gram-positive infections. Telavancin is a semi-synthetic derivative of vancomycin. The FDA approved the drug in September 2009 for complicated skin and skin structure infections (cSSSI), and in June 2013 for hospital-acquired and ventilator-associated bacterial pneumonia caused by Staphylococcus aureus. History On 19 October 2007, the US Food and Drug Administration (FDA) issued an approvable letter for telavancin. Its developer, Theravance, submitted a complete response to the letter, and the FDA has assigned a Prescription Drug User Fee Act (PDUFA) target date of 21 July 2008. On 19 November 2008, an FDA antiinfective drug advisory committee concluded that they would recommend telavancin be approved by the FDA. The FDA approved the drug on 11 September 2009 for complicated skin and skin structure infections ( cSSSI). Theravance has also submitted telavancin to the FDA in a secon ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oritavancin
Oritavancin, sold under the brand name Orbactiv among others, is a semisynthetic glycopeptide antibiotic medication for the treatment of serious Gram-positive bacterial infections. Its chemical structure as a lipoglycopeptide is similar to vancomycin. The U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA) have approved oritavancin for treatment of acute bacterial ABSSSI, skin and skin structure infections. ''In vitro'' activity Oritavancin shares certain properties with other members of the glycopeptide class of antibiotics, which includes vancomycin, the current standard of care for serious Gram-positive infections in the United States and Europe. It possesses potent and rapid bactericidal activity ''in vitro'' against a broad spectrum of both resistant and susceptible Gram-positive bacteria, including ''Staphylococcus aureus'', methicillin-resistant Staphylococcus aureus, MRSA, enterococci, and streptococci. Oritavancin was more active than ei ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Antibiotic
An antibiotic is a type of antimicrobial substance active against bacteria. It is the most important type of antibacterial agent for fighting bacterial infections, and antibiotic medications are widely used in the treatment and prevention of such infections. They may either kill or inhibit the growth of bacteria. A limited number of antibiotics also possess antiprotozoal activity. Antibiotics are not effective against viruses such as the common cold or influenza; drugs which inhibit viruses are termed antiviral drugs or antivirals rather than antibiotics. Sometimes, the term ''antibiotic''—literally "opposing life", from the Greek roots ἀντι ''anti'', "against" and βίος ''bios'', "life"—is broadly used to refer to any substance used against microbes, but in the usual medical usage, antibiotics (such as penicillin) are those produced naturally (by one microorganism fighting another), whereas non-antibiotic antibacterials (such as sulfonamides and antiseptics) ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oritavancin
Oritavancin, sold under the brand name Orbactiv among others, is a semisynthetic glycopeptide antibiotic medication for the treatment of serious Gram-positive bacterial infections. Its chemical structure as a lipoglycopeptide is similar to vancomycin. The U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA) have approved oritavancin for treatment of acute bacterial ABSSSI, skin and skin structure infections. ''In vitro'' activity Oritavancin shares certain properties with other members of the glycopeptide class of antibiotics, which includes vancomycin, the current standard of care for serious Gram-positive infections in the United States and Europe. It possesses potent and rapid bactericidal activity ''in vitro'' against a broad spectrum of both resistant and susceptible Gram-positive bacteria, including ''Staphylococcus aureus'', methicillin-resistant Staphylococcus aureus, MRSA, enterococci, and streptococci. Oritavancin was more active than ei ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Telavancin
Telavancin (trade name Vibativ) is a bactericidal lipoglycopeptide for use in MRSA or other Gram-positive infections. Telavancin is a semi-synthetic derivative of vancomycin. The FDA approved the drug in September 2009 for complicated skin and skin structure infections (cSSSI), and in June 2013 for hospital-acquired and ventilator-associated bacterial pneumonia caused by Staphylococcus aureus. History On 19 October 2007, the US Food and Drug Administration (FDA) issued an approvable letter for telavancin. Its developer, Theravance, submitted a complete response to the letter, and the FDA has assigned a Prescription Drug User Fee Act (PDUFA) target date of 21 July 2008. On 19 November 2008, an FDA antiinfective drug advisory committee concluded that they would recommend telavancin be approved by the FDA. The FDA approved the drug on 11 September 2009 for complicated skin and skin structure infections ( cSSSI). Theravance has also submitted telavancin to the FDA in a secon ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Antibiotic
An antibiotic is a type of antimicrobial substance active against bacteria. It is the most important type of antibacterial agent for fighting bacterial infections, and antibiotic medications are widely used in the treatment and prevention of such infections. They may either kill or inhibit the growth of bacteria. A limited number of antibiotics also possess antiprotozoal activity. Antibiotics are not effective against viruses such as the common cold or influenza; drugs which inhibit viruses are termed antiviral drugs or antivirals rather than antibiotics. Sometimes, the term ''antibiotic''—literally "opposing life", from the Greek roots ἀντι ''anti'', "against" and βίος ''bios'', "life"—is broadly used to refer to any substance used against microbes, but in the usual medical usage, antibiotics (such as penicillin) are those produced naturally (by one microorganism fighting another), whereas non-antibiotic antibacterials (such as sulfonamides and antiseptics) ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Qualified Infectious Disease Product
The Food and Drug Administration Safety and Innovation Act of 2012 (FDASIA) is a piece of American regulatory legislation signed into law on July 9, 2012. It gives the United States Food and Drug Administration (FDA) the authority to collect user fees from the medical industry to fund reviews of innovator drugs, medical devices, generic drugs and biosimilar biologics. It also creates the breakthrough therapy designation program and extends the priority review voucher program to make eligible rare pediatric diseases. The measure was passed by 96 senators voting for and one voting against. Title I: Fees Relating to Drugs Title I extends through FY2017 the authority of the FDA, through the authority of the Secretary of Health and Human Services, to collect drug application and supplement fees, prescription drug establishment fees, and prescription drug product fees to support the FDA process for reviewing human drug applications. It requires the FDA to submit annually to the House ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Streptococcus Pyogenes
''Streptococcus pyogenes'' is a species of Gram-positive, aerotolerant bacteria in the genus ''Streptococcus''. These bacteria are extracellular, and made up of non-motile and non-sporing cocci (round cells) that tend to link in chains. They are clinically important for humans, as they are an infrequent, but usually pathogenic, part of the skin microbiota that can cause Group A streptococcal infection. ''S. pyogenes'' is the predominant species harboring the Lancefield group A antigen, and is often called group A ''Streptococcus'' (GAS). However, both '' Streptococcus dysgalactiae'' and the '' Streptococcus anginosus'' group can possess group A antigen as well. Group A streptococci, when grown on blood agar, typically produce small (2–3 mm) zones of beta-hemolysis, a complete destruction of red blood cells. The name group A (beta-hemolytic) ''Streptococcus'' (GABHS) is thus also used. The species name is derived from Greek words meaning 'a chain' () of berries ( a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
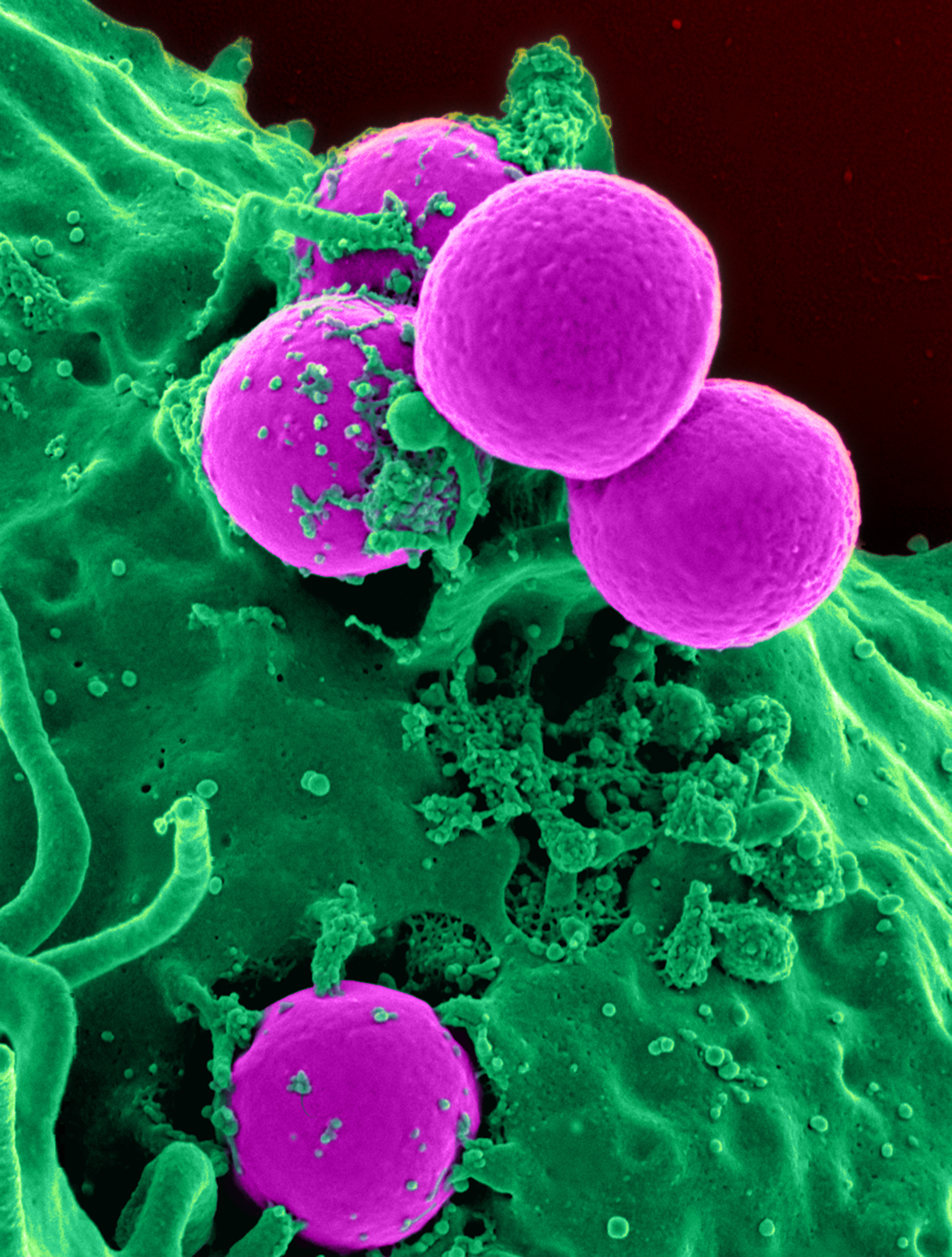
Staphylococcus Aureus
''Staphylococcus aureus'' is a Gram-positive spherically shaped bacterium, a member of the Bacillota, and is a usual member of the microbiota of the body, frequently found in the upper respiratory tract and on the skin. It is often positive for catalase and nitrate reduction and is a facultative anaerobe that can grow without the need for oxygen. Although ''S. aureus'' usually acts as a commensal of the human microbiota, it can also become an opportunistic pathogen, being a common cause of skin infections including abscesses, respiratory infections such as sinusitis, and food poisoning. Pathogenic strains often promote infections by producing virulence factors such as potent protein toxins, and the expression of a cell-surface protein that binds and inactivates antibodies. ''S. aureus'' is one of the leading pathogens for deaths associated with antimicrobial resistance and the emergence of antibiotic-resistant strains, such as methicillin-resistant ''S. aureus'' (MRSA ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
MRSA
Methicillin-resistant ''Staphylococcus aureus'' (MRSA) is a group of Gram-positive bacteria that are genetically distinct from other strains of ''Staphylococcus aureus''. MRSA is responsible for several difficult-to-treat infections in humans. It caused more than 100,000 deaths attributable to antimicrobial resistance in 2019. MRSA is any strain of ''S. aureus'' that has developed (through natural selection) or acquired (through horizontal gene transfer) a multiple drug resistance to beta-lactam antibiotics. Beta-lactam (β-lactam) antibiotics are a broad-spectrum group that include some penams (penicillin derivatives such as methicillin and oxacillin) and cephems such as the cephalosporins. Strains unable to resist these antibiotics are classified as methicillin-susceptible ''S. aureus'', or MSSA. MRSA is common in hospitals, prisons, and nursing homes, where people with open wounds, invasive devices such as catheters, and weakened immune systems are at greater risk of hea ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |