|
Levinthal's Paradox
Levinthal's paradox is a thought experiment, also constituting a self-reference in the theory of protein folding. In 1969, Cyrus Levinthal noted that, because of the very large number of degrees of freedom in an unfolded polypeptide chain, the molecule has an astronomical number of possible conformations. An estimate of 10300 was made in one of his papers (often incorrectly cited as the 1968 paper). For example, a polypeptide of 100 residues will have 99 peptide bonds, and therefore 198 different phi and psi bond angles. If each of these bond angles can be in one of three stable conformations, the protein may misfold into a maximum of 3198 different conformations (including any possible folding redundancy). Therefore, if a protein were to attain its correctly folded configuration by sequentially sampling all the possible conformations, it would require a time longer than the age of the universe to arrive at its correct native conformation. This is true even if conformations are ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thought Experiment
A thought experiment is a hypothetical situation in which a hypothesis, theory, or principle is laid out for the purpose of thinking through its consequences. History The ancient Greek ''deiknymi'' (), or thought experiment, "was the most ancient pattern of mathematical proof", and existed before Euclidean mathematics, where the emphasis was on the conceptual, rather than on the experimental part of a thought-experiment. Johann Witt-Hansen established that Hans Christian Ørsted was the first to use the German term ' (lit. thought experiment) circa 1812. Ørsted was also the first to use the equivalent term ' in 1820. By 1883 Ernst Mach used the term ' in a different way, to denote exclusively the conduct of a experiment that would be subsequently performed as a by his students. Physical and mental experimentation could then be contrasted: Mach asked his students to provide him with explanations whenever the results from their subsequent, real, physical experiment differed ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protein Structure Prediction
Protein structure prediction is the inference of the three-dimensional structure of a protein from its amino acid sequence—that is, the prediction of its secondary and tertiary structure from primary structure. Structure prediction is different from the inverse problem of protein design. Protein structure prediction is one of the most important goals pursued by computational biology; and it is important in medicine (for example, in drug design) and biotechnology (for example, in the design of novel enzymes). Starting in 1994, the performance of current methods is assessed biannually in the CASP experiment (Critical Assessment of Techniques for Protein Structure Prediction). A continuous evaluation of protein structure prediction web servers is performed by the community project CAMEO3D. Protein structure and terminology Proteins are chains of amino acids joined together by peptide bonds. Many conformations of this chain are possible due to the rotation of the main chain abou ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protein Structure
Protein structure is the three-dimensional arrangement of atoms in an amino acid-chain molecule. Proteins are polymers specifically polypeptides formed from sequences of amino acids, the monomers of the polymer. A single amino acid monomer may also be called a ''residue'' indicating a repeating unit of a polymer. Proteins form by amino acids undergoing condensation reactions, in which the amino acids lose one water molecule per reaction in order to attach to one another with a peptide bond. By convention, a chain under 30 amino acids is often identified as a peptide, rather than a protein. To be able to perform their biological function, proteins fold into one or more specific spatial conformations driven by a number of non-covalent interactions such as hydrogen bonding, ionic interactions, Van der Waals forces, and hydrophobic packing. To understand the functions of proteins at a molecular level, it is often necessary to determine their three-dimensional structure. This is t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chaperone (protein)
In molecular biology, molecular chaperones are proteins that assist the conformational folding or unfolding of large proteins or macromolecular protein complexes. There are a number of classes of molecular chaperones, all of which function to assist large proteins in proper protein folding during or after synthesis, and after partial denaturation. Chaperones are also involved in the translocation of proteins for proteolysis. The first molecular chaperones discovered were a type of assembly chaperones which assist in the assembly of nucleosomes from folded histones and DNA. One major function of molecular chaperones is to prevent the aggregation of misfolded proteins, thus many chaperone proteins are classified as heat shock proteins, as the tendency for protein aggregation is increased by heat stress. The majority of molecular chaperones do not convey any steric information for protein folding, and instead assist in protein folding by binding to and stabilizing folding interme ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Edward Trifonov
Edward Nikolayevich Trifonov ( he, אדוארד טריפונוב, russian: Эдуapд Тpифoнoв; b. March 31, 1937) is a Russian-born Israeli molecular biophysicist and a founder of Israeli bioinformatics. In his research, he specializes in the recognition of weak signal patterns in biological sequences and is known for his unorthodox scientific methods. He discovered the 3-bp and 10-bp periodicity in the DNA sequences, as well as the rules determining the curvature of DNA molecules and their bending within nucleosomes. Trifonov unveiled multiple novel codes in biological sequences and the modular structure of proteins. He proposed an abiogenic theory of the origin of life, and molecular evolution from single nucleotides and amino acids to present-day DNA and protein sequences. Biography Trifonov was born in Leningrad (now Saint Petersburg), USSR in 1937. He was raised by his mother, Riva, and his step-father, Nikolay Nikolayevich Trifonov. In his school years, he becam ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Folding Funnel
The folding funnel hypothesis is a specific version of the energy landscape theory of protein folding, which assumes that a protein's native state corresponds to its free energy minimum under the solution conditions usually encountered in cells. Although energy landscapes may be "rough", with many non-native local minima in which partially folded proteins can become trapped, the folding funnel hypothesis assumes that the native state is a deep free energy minimum with steep walls, corresponding to a single well-defined tertiary structure. The term was introduced by Ken A. Dill in a 1987 article discussing the stabilities of globular proteins. The folding funnel hypothesis is closely related to the hydrophobic collapse hypothesis, under which the driving force for protein folding is the stabilization associated with the sequestration of hydrophobic amino acid side chains in the interior of the folded protein. This allows the water solvent to maximize its entropy, lowering the tot ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
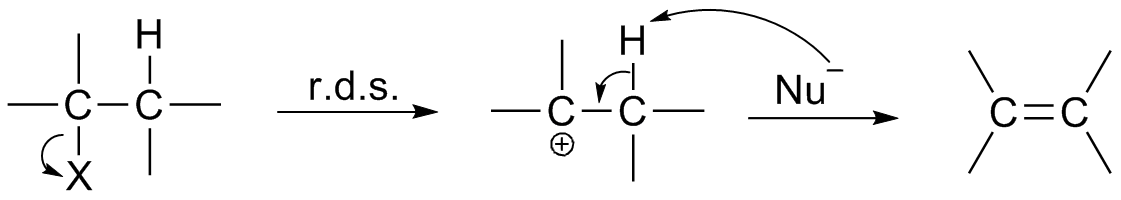
Transition State
In chemistry, the transition state of a chemical reaction is a particular configuration along the reaction coordinate. It is defined as the state corresponding to the highest potential energy along this reaction coordinate. It is often marked with the double dagger ‡ symbol. As an example, the transition state shown below occurs during the SN2 reaction of bromoethane with a hydroxide anion: The activated complex of a reaction can refer to either the transition state or to other states along the reaction coordinate between reactants and products, especially those close to the transition state.Peter Atkins and Julio de Paula, ''Physical Chemistry'' (8th ed., W.H. Freeman 2006), p.809 According to the transition state theory, once the reactants have passed through the transition state configuration, they always continue to form products. History of concept The concept of a transition state has been important in many theories of the rates at which chemical reactions occ ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Reaction Intermediate
In chemistry, a reaction intermediate or an intermediate is a molecular entity that is formed from the reactants (or preceding intermediates) but is consumed in further reactions in stepwise chemical reactions that contain multiple elementary steps. Intermediates are the reaction product of one elementary step, but do not appear in the chemical equation for an overall chemical equation. For example, consider this hypothetical stepwise reaction: :A + B -> C + D The reaction includes two elementary steps: :A + B -> X :X -> C + D In this example, X is a reaction intermediate. IUPAC definition The IUPAC Gold Book defines an ''intermediate'' as a compound that has a lifetime greater than a molecular vibration that is formed (directly or indirectly) from the reactants and reacts further to give (either directly or indirectly) the products of a chemical reaction. The lifetime condition distinguishes true, chemically distinct intermediates from vibrational states or such transition st ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nucleation
In thermodynamics, nucleation is the first step in the formation of either a new thermodynamic phase or structure via self-assembly or self-organization within a substance or mixture. Nucleation is typically defined to be the process that determines how long an observer has to wait before the new phase or self-organized structure appears. For example, if a volume of water is cooled (at atmospheric pressure) below 0°C, it will tend to freeze into ice, but volumes of water cooled only a few degrees below 0°C often stay completely free of ice for long periods (supercooling). At these conditions, nucleation of ice is either slow or does not occur at all. However, at lower temperatures nucleation is fast, and ice crystals appear after little or no delay. Nucleation is a common mechanism which generates first-order phase transitions, and it is the start of the process of forming a new thermodynamic phase. In contrast, new phases at continuous phase transitions start to form immedi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Anfinsen's Dogma
Anfinsen's dogma, also known as the thermodynamic hypothesis, is a postulate in molecular biology. It states that, at least for a small globular protein in its standard physiological environment, the native structure is determined only by the protein's amino acid sequence. The dogma was championed by the Nobel Prize Laureate Christian B. Anfinsen from his research on the folding of ribonuclease A. The postulate amounts to saying that, at the environmental conditions (temperature, solvent concentration and composition, etc.) at which folding occurs, the native structure is a unique, stable and kinetically accessible minimum of the free energy. In other words, there are three conditions for formation of a unique protein structure: *Uniqueness – Requires that the sequence does not have any other configuration with a comparable free energy. Hence the free energy minimum must be ''unchallenged''. *Stability – Small changes in the surrounding environment cannot give rise to changes ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Picosecond
A picosecond (abbreviated as ps) is a unit of time in the International System of Units (SI) equal to 10−12 or (one trillionth) of a second. That is one trillionth, or one millionth of one millionth of a second, or 0.000 000 000 001 seconds. A picosecond is to one second as one second is to approximately 31,689 years. Multiple technical approaches achieve imaging within single-digit picoseconds: for example, the streak camera or intensified CCD (ICCD) cameras are able to picture the motion of light. One picosecond is equal to 1000 femtoseconds, or 1/1000 nanoseconds. Because the next SI unit is 1000 times larger, measurements of 10−11 and 10−10 second are typically expressed as tens or hundreds of picoseconds. Some notable measurements in this range include: * 1.0 picoseconds (1.0 ps) – cycle time for electromagnetic frequency 1 terahertz (THz) (1 x 1012 hertz), an inverse unit. This corresponds to a wavelength of 0.3 mm, as can be calculated by m ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Self-reference
Self-reference occurs in natural or formal languages when a sentence, idea or formula refers to itself. The reference may be expressed either directly—through some intermediate sentence or formula—or by means of some encoding. In philosophy, it also refers to the ability of a subject to speak of or refer to itself, that is, to have the kind of thought expressed by the first person nominative singular pronoun "I" in English. Self-reference is studied and has applications in mathematics, philosophy, computer programming, second-order cybernetics, and linguistics, as well as in humor. Self-referential statements are sometimes paradoxical, and can also be considered recursive. In logic, mathematics and computing In classical philosophy, paradoxes were created by self-referential concepts such as the omnipotence paradox of asking if it was possible for a being to exist so powerful that it could create a stone that it could not lift. The Epimenides paradox, 'All Cretans are ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |