|
Lead And Zinc Mines In Japan
Lead is a chemical element with the symbol Pb (from the Latin ) and atomic number 82. It is a heavy metal that is denser than most common materials. Lead is soft and malleable, and also has a relatively low melting point. When freshly cut, lead is a shiny gray with a hint of blue. It tarnishes to a dull gray color when exposed to air. Lead has the highest atomic number of any stable element and three of its isotopes are endpoints of major nuclear decay chains of heavier elements. Lead is toxic, even in small amounts, especially to children. Lead is a relatively unreactive post-transition metal. Its weak metallic character is illustrated by its amphoteric nature; lead and lead oxides react with acids and bases, and it tends to form covalent bonds. Compounds of lead are usually found in the +2 oxidation state rather than the +4 state common with lighter members of the carbon group. Exceptions are mostly limited to organolead compounds. Like the lighter members of the group, lea ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Element
A chemical element is a species of atoms that have a given number of protons in their nuclei, including the pure substance consisting only of that species. Unlike chemical compounds, chemical elements cannot be broken down into simpler substances by any chemical reaction. The number of protons in the nucleus is the defining property of an element, and is referred to as its atomic number (represented by the symbol ''Z'') – all atoms with the same atomic number are atoms of the same element. Almost all of the baryonic matter of the universe is composed of chemical elements (among rare exceptions are neutron stars). When different elements undergo chemical reactions, atoms are rearranged into new compounds held together by chemical bonds. Only a minority of elements, such as silver and gold, are found uncombined as relatively pure native element minerals. Nearly all other naturally occurring elements occur in the Earth as compounds or mixtures. Air is primarily a mixture o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Covalent Bond
A covalent bond is a chemical bond that involves the sharing of electrons to form electron pairs between atoms. These electron pairs are known as shared pairs or bonding pairs. The stable balance of attractive and repulsive forces between atoms, when they share electrons, is known as covalent bonding. For many molecules, the sharing of electrons allows each atom to attain the equivalent of a full valence shell, corresponding to a stable electronic configuration. In organic chemistry, covalent bonding is much more common than ionic bonding. Covalent bonding also includes many kinds of interactions, including σ-bonding, π-bonding, metal-to-metal bonding, agostic interactions, bent bonds, three-center two-electron bonds and three-center four-electron bonds. The term ''covalent bond'' dates from 1939. The prefix ''co-'' means ''jointly, associated in action, partnered to a lesser degree, '' etc.; thus a "co-valent bond", in essence, means that the atoms share " valence", such a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ductility
Ductility is a mechanical property commonly described as a material's amenability to drawing (e.g. into wire). In materials science, ductility is defined by the degree to which a material can sustain plastic deformation under tensile stress before failure. Ductility is an important consideration in engineering and manufacturing. It defines a material's suitability for certain manufacturing operations (such as cold working) and its capacity to absorb mechanical overload.. Some metals that are generally described as ductile include gold and copper. However, not all metals experience ductile failure as some can be characterized with brittle failure like cast iron. Polymers generally can be viewed as ductile materials as they typically allow for plastic deformation. Malleability, a similar mechanical property, is characterized by a material's ability to deform plastically without failure under compressive stress. Historically, materials were considered malleable if they were am ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Movable Type
Movable type (US English; moveable type in British English) is the system and technology of printing and typography that uses movable components to reproduce the elements of a document (usually individual alphanumeric characters or punctuation marks) usually on the medium of paper. The world's first movable type printing technology for paper books was made of porcelain materials and was invented around AD 1040 in China during the Northern Song dynasty by the inventor Bi Sheng (990–1051). The earliest printed paper money with movable metal type to print the identifying code of the money was made in 1161 during the Song dynasty. In 1193, a book in the Song dynasty documented how to use the copper movable type. The oldest extant book printed with movable metal type, Jikji, was printed in Korea in 1377 during the Goryeo dynasty. The spread of both movable-type systems was, to some degree, limited to primarily East Asia. The development of the printing press in Europe may have ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Printing Press
A printing press is a mechanical device for applying pressure to an inked surface resting upon a printing, print medium (such as paper or cloth), thereby transferring the ink. It marked a dramatic improvement on earlier printing methods in which the cloth, paper or other medium was brushed or rubbed repeatedly to achieve the transfer of ink, and accelerated the process. Typically used for texts, the invention and global spread of the printing press was one of the most influential events in the second millennium. In Germany, around 1440, goldsmith Johannes Gutenberg invented the movable-type printing press, which started the Printing Revolution. Modelled on the design of existing screw presses, a single Renaissance movable-type printing press could produce up to 3,600 pages per workday, compared to forty by History of typography in East Asia, hand-printing and a few by scribe, hand-copying. Gutenberg's newly devised matrix (printing), hand mould made possible the precise and ra ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Industrial Revolution
The Industrial Revolution was the transition to new manufacturing processes in Great Britain, continental Europe, and the United States, that occurred during the period from around 1760 to about 1820–1840. This transition included going from hand production methods to machines, new chemical manufacturing and iron production processes, the increasing use of steam power and water power, the development of machine tools and the rise of the mechanized factory system. Output greatly increased, and a result was an unprecedented rise in population and in the rate of population growth. Textiles were the dominant industry of the Industrial Revolution in terms of employment, value of output and capital invested. The textile industry was also the first to use modern production methods. The Industrial Revolution began in Great Britain, and many of the technological and architectural innovations were of British origin. By the mid-18th century, Britain was the world's leadi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fall Of The Western Roman Empire
The fall of the Western Roman Empire (also called the fall of the Roman Empire or the fall of Ancient Rome, Rome) was the loss of central political control in the Western Roman Empire, a process in which the Empire failed to enforce its rule, and its vast territory was divided into several successor Polity, polities. The Roman Empire lost the strengths that had allowed it to exercise effective control over its Western Roman province, provinces; modern historians posit factors including the effectiveness and numbers of the Late Roman army, army, the health and numbers of the Roman population, the strength of the Roman economy, economy, the competence of the Roman emperor, emperors, the internal struggles for power, the religious changes of the period, and the efficiency of the civil administration. Increasing pressure from invading barbarians outside Roman culture also contributed greatly to the collapse. Climate variability and change, Climatic changes and both Endemic (epide ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
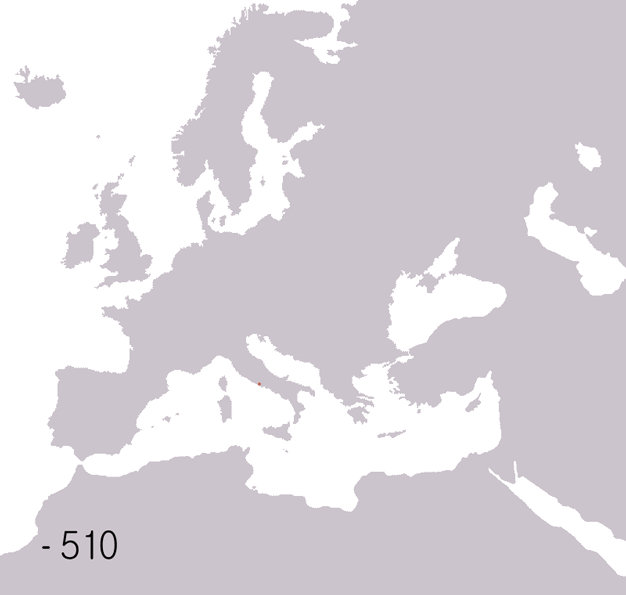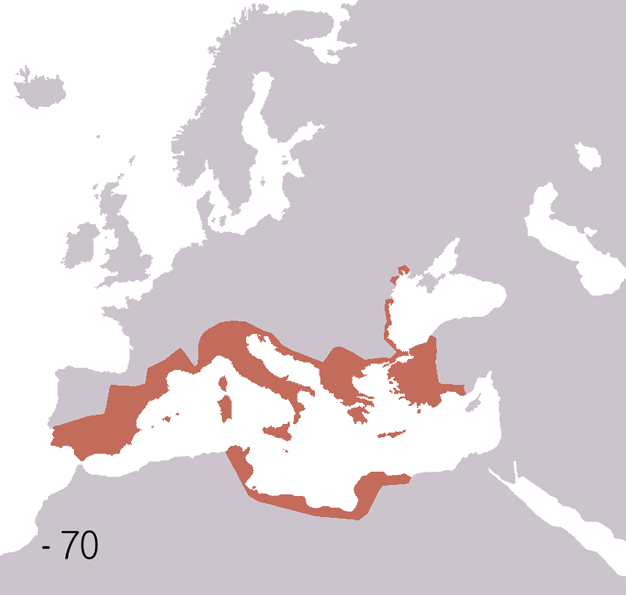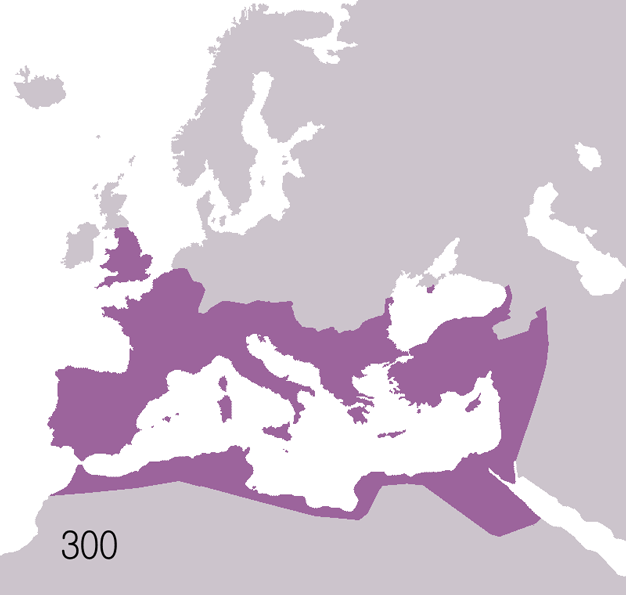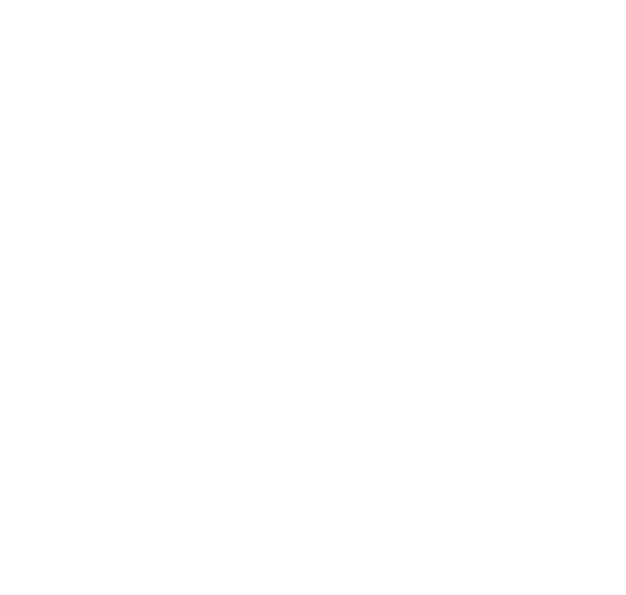
Ancient Rome
In modern historiography, ancient Rome refers to Roman civilisation from the founding of the city of Rome in the 8th century BC to the collapse of the Western Roman Empire in the 5th century AD. It encompasses the Roman Kingdom (753–509 BC), Roman Republic (509–27 BC) and Roman Empire (27 BC–476 AD) until the fall of the western empire. Ancient Rome began as an Italic settlement, traditionally dated to 753 BC, beside the River Tiber in the Italian Peninsula. The settlement grew into the city and polity of Rome, and came to control its neighbours through a combination of treaties and military strength. It eventually dominated the Italian Peninsula, assimilated the Greek culture of southern Italy ( Magna Grecia) and the Etruscan culture and acquired an Empire that took in much of Europe and the lands and peoples surrounding the Mediterranean Sea. It was among the largest empires in the ancient world, with an estimated 50 to 90 million inhabitants, roughly 20% of t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Galena
Galena, also called lead glance, is the natural mineral form of lead(II) sulfide (PbS). It is the most important ore of lead and an important source of silver. Galena is one of the most abundant and widely distributed sulfide minerals. It crystallizes in the cubic crystal system often showing octahedral forms. It is often associated with the minerals sphalerite, calcite and fluorite. Occurrence Galena is the main ore of lead, used since ancient times, since lead can be smelted from galena in an ordinary wood fire. Galena typically is found in hydrothermal veins in association with sphalerite, marcasite, chalcopyrite, cerussite, anglesite, dolomite, calcite, quartz, barite, and fluorite. It is also found in association with sphalerite in low-temperature lead-zinc deposits within limestone beds. Minor amounts are found in contact metamorphic zones, in pegmatites, and disseminated in sedimentary rock. In some deposits the galena contains up to 0.5% silver, a byproduct that ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Metals Of Antiquity
The metals of antiquity are the seven metals which humans had identified and found use for in prehistoric times in Europe and the Middle East: gold, silver, copper, tin, lead, iron, and mercury. These seven are the metals from which the modern world was forged; until the discovery of antimony in the 9th century,George Sarton, ''Introduction to the History of Science''. "We find in his writings ..preparation of various substances (e.g., basic lead carbonatic, arsenic and antimony from their sulphides)." and arsenic in the 13th (both now classified as metalloids), these were the only known elemental metals, compared to approximately 90 known today. Characteristics Melting point The metals of antiquity generally have low melting points, with iron being the exception. * Mercury melts at −38.829 °C (-37.89 °F) (being liquid at room temperature). * Tin melts at 231 °C (449 °F) * Lead melts at 327 °C (621 °F) * Silver at 961 °C (1763&nb ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Catenation
In chemistry, catenation is the bonding of atoms of the same element into a series, called a ''chain''. A chain or a ring shape may be ''open'' if its ends are not bonded to each other (an open-chain compound), or ''closed'' if they are bonded in a ring (a cyclic compound). The words ''to catenate'' and ''catenation'' reflect the Latin root '' catena'', "chain". Carbon Catenation occurs most readily with carbon, which forms covalent bonds with other carbon atoms to form longer chains and structures. This is the reason for the presence of the vast number of organic compounds in nature. Carbon is most well known for its properties of catenation, with organic chemistry essentially being the study of catenated carbon structures (and known as catenae). Carbon chains in biochemistry combine any of various other elements, such as hydrogen, oxygen, and biometals, onto the backbone of carbon. However, carbon is by no means the only element capable of forming such catenae, and several ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organolead Compound
Organolead compounds are chemical compounds containing a chemical bond between carbon and lead. Organolead chemistry is the corresponding science. The first organolead compound was hexaethyldilead (Pb2(C2H5)6), first synthesized in 1858.''Main Group Metals in Organic Synthesis'' Yamamoto, Hisashi / Oshima, Koichiro (eds.) 2004 Sharing the same group with carbon, lead is tetravalent. Going down the carbon group the C–''X'' (''X'' = C, Si, Ge, Sn, Pb) bond becomes weaker and the bond length larger. The C–Pb bond in tetramethyllead is 222 pm long with a dissociation energy of 49 kcal/ mol (204 kJ/mol). For comparison the C–Sn bond in tetramethyltin is 214 pm long with dissociation energy 71 kcal/mol (297 kJ/mol). The dominance of Pb(IV) in organolead chemistry is remarkable because inorganic lead compounds tend to have Pb(II) centers. The reason is that with inorganic lead compounds elements such as nitrogen, oxygen and the halides have a much higher electronegativity than le ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |

%2C_black_and_white.png)