|
Lead(II) Selenide
Lead selenide (PbSe), or lead(II) selenide, a selenide of lead, is a semiconductor material. It forms cubic crystals of the NaCl structure; it has a direct bandgap of 0.27 eV at room temperature. (Note that incorrectly identifies PbSe and other IV–VI semiconductors as indirect gap materials.) A grey solid, it is used for manufacture of infrared detectors for thermal imaging. The mineral clausthalite is a naturally occurring lead selenide. It may be formed by direct reaction between its constituent elements, lead and selenium. Infrared detection PbSe was one of the first materials found to be sensitive to the infrared radiation used for military applications. Early research works on the material as infrared detector were carried out during the 1930s and the first useful devices were processed by Germans, Americans and British during and just after World War II. Since then, PbSe has been commonly used as an infrared photodetector in multiple applications, from spectrom ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Clausthalite
Clausthalite is a lead selenide mineral, PbSe. It forms a solid solution series with galena PbS. Occurrence It occurs in low-sulfur hydrothermal deposits with other selenides and in mercury deposits. It is associated with tiemannite, klockmannite, berzelianite, umangite, gold, stibiopalladinite and uraninite. It was first described in 1832 and named for the discovery locality of Clausthal-Zellerfeld in the Harz Mountains, Germany. See also * Classification of minerals * List of minerals This is a list of minerals for which there are articles on Wikipedia. Minerals are distinguished by various chemical and physical properties. Differences in chemical composition and crystal structure distinguish the various ''species''. Within a m ... References Lead minerals Selenide minerals Galena group {{mineral-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Direct Bandgap
In semiconductor physics, the band gap of a semiconductor can be of two basic types, a direct band gap or an indirect band gap. The minimal-energy state in the conduction band and the maximal-energy state in the valence band are each characterized by a certain crystal momentum (k-vector) in the Brillouin zone. If the k-vectors are different, the material has an "indirect gap". The band gap is called "direct" if the crystal momentum of electrons and holes is the same in both the conduction band and the valence band; an electron can directly emit a photon. In an "indirect" gap, a photon cannot be emitted because the electron must pass through an intermediate state and transfer momentum to the crystal lattice. Examples of direct bandgap materials include amorphous silicon and some III-V materials such as InAs and GaAs. Indirect bandgap materials include crystalline silicon and Ge. Some III-V materials are indirect bandgap as well, for example AlSb. Implications for radiati ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Photoconductor
Photoconductivity is an optical and electrical phenomenon in which a material becomes more electrically conductive due to the absorption of electromagnetic radiation such as visible light, ultraviolet light, infrared light, or gamma radiation. When light is absorbed by a material such as a semiconductor, the number of free electrons and holes increases, resulting in increased electrical conductivity. To cause excitation, the light that strikes the semiconductor must have enough energy to raise electrons across the band gap, or to excite the impurities within the band gap. When a bias voltage and a load resistor are used in series with the semiconductor, a voltage drop across the load resistors can be measured when the change in electrical conductivity of the material varies the current through the circuit. Classic examples of photoconductive materials include: * photographic film: Kodachrome, Fujifilm, Agfachrome, Ilford, ''etc.'', based on silver sulfide and silver bromide. * the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
MWIR
Infrared (IR), sometimes called infrared light, is electromagnetic radiation (EMR) with wavelengths longer than those of Light, visible light. It is therefore invisible to the human eye. IR is generally understood to encompass wavelengths from around 1 millimeter (300 GHz) to the nominal red edge of the visible spectrum, around 700 nanometers (430 Terahertz (unit), THz). Longer IR wavelengths (30 μm-100 μm) are sometimes included as part of the terahertz radiation range. Almost all black-body radiation from objects near room temperature is at infrared wavelengths. As a form of electromagnetic radiation, IR propagates energy and momentum, exerts radiation pressure, and has properties corresponding to Wave–particle duality, both those of a wave and of a Subatomic particle, particle, the photon. It was long known that fires emit invisible heat; in 1681 the pioneering experimenter Edme Mariotte showed that glass, though transparent to sunlight, obstructed rad ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
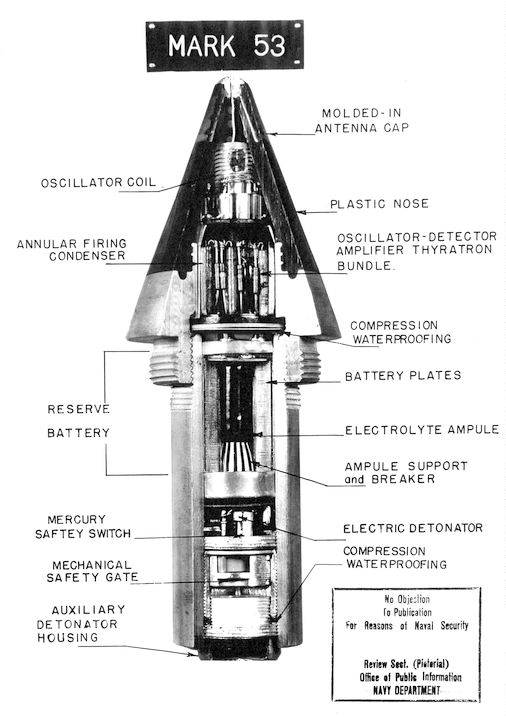
Fuze
In military munitions, a fuze (sometimes fuse) is the part of the device that initiates function. In some applications, such as torpedoes, a fuze may be identified by function as the exploder. The relative complexity of even the earliest fuze designs can be seen in cutaway diagrams. A fuze is a device that detonates a munition's explosive material under specified conditions. In addition, a fuze will have safety and arming mechanisms that protect users from premature or accidental detonation. For example, an artillery fuze's battery is activated by the high acceleration of cannon launch, and the fuze must be spinning rapidly before it will function. "Complete bore safety" can be achieved with mechanical shutters that isolate the detonator from the main charge until the shell is fired. A fuze may contain only the electronic or mechanical elements necessary to signal or actuate the detonator, but some fuzes contain a small amount of primary explosive to initiate the detonation. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Flame
A flame (from Latin ''flamma'') is the visible, gaseous part of a fire. It is caused by a highly exothermic chemical reaction taking place in a thin zone. When flames are hot enough to have ionized gaseous components of sufficient density they are then considered plasma. Mechanism Color and temperature of a flame are dependent on the type of fuel involved in the combustion, as, for example, when a lighter is held to a candle. The applied heat causes the fuel molecules in the candle wax to vaporize (if this process happens in inert atmosphere without oxidizer, it is called pyrolysis). In this state they can then readily react with oxygen in the air, which gives off enough heat in the subsequent exothermic reaction to vaporize yet more fuel, thus sustaining a consistent flame. The high temperature of the flame causes the vaporized fuel molecules to decompose, forming various incomplete combustion products and free radicals, and these products then react with each other and with ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Spectrometers
A spectrometer () is a scientific instrument used to separate and measure spectral components of a physical phenomenon. Spectrometer is a broad term often used to describe instruments that measure a continuous variable of a phenomenon where the spectral components are somehow mixed. In visible light a spectrometer can separate white light and measure individual narrow bands of color, called a spectrum. A mass spectrometer measures the spectrum of the masses of the atoms or molecules present in a gas. The first spectrometers were used to split light into an array of separate colors. Spectrometers were developed in early studies of physics, astronomy, and chemistry. The capability of spectroscopy to determine chemical composition drove its advancement and continues to be one of its primary uses. Spectrometers are used in astronomy to analyze the chemical composition of stars and planets, and spectrometers gather data on the origin of the universe. Examples of spectrometers are d ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
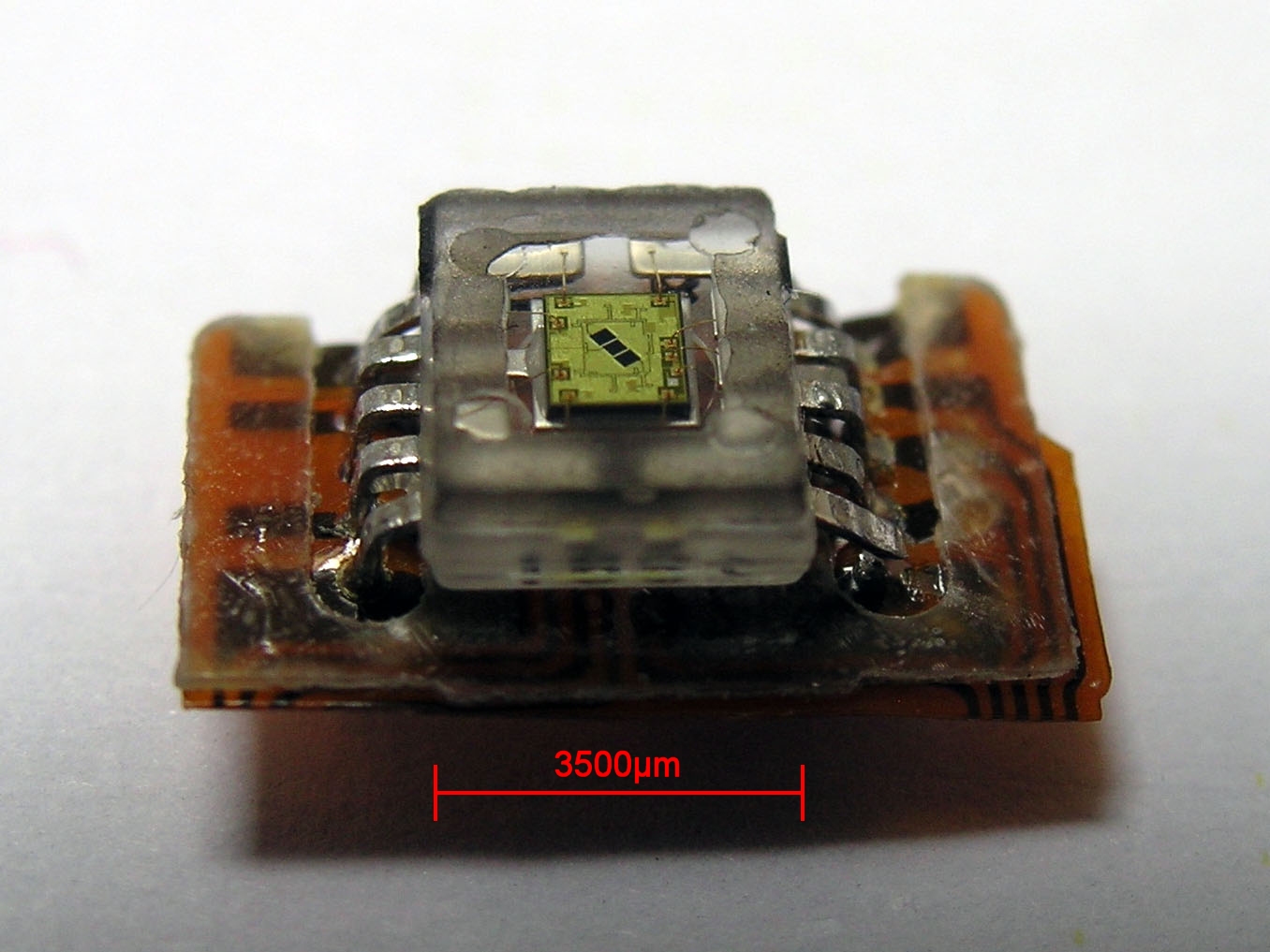
Photodetector
Photodetectors, also called photosensors, are sensors of light or other electromagnetic radiation. There is a wide variety of photodetectors which may be classified by mechanism of detection, such as Photoelectric effect, photoelectric or photochemical effects, or by various performance metrics, such as spectral response. Semiconductor-based photodetectors typically photo detector have a p–n junction that converts light photons into current. The absorbed photons make electron–hole pairs in the depletion region. Photodiodes and photo transistors are a few examples of photo detectors. Solar cells convert some of the light energy absorbed into electrical energy. Types Photodetectors may be classified by their mechanism for detection: * Photoemission or photoelectric effect: Photons cause electrons to transition from the conduction band of a material to free electrons in a vacuum or gas. * Thermal: Photons cause electrons to transition to mid-gap states then decay back to lo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Infrared
Infrared (IR), sometimes called infrared light, is electromagnetic radiation (EMR) with wavelengths longer than those of visible light. It is therefore invisible to the human eye. IR is generally understood to encompass wavelengths from around 1 millimeter (300 GHz) to the nominal red edge of the visible spectrum, around 700 nanometers (430 THz). Longer IR wavelengths (30 μm-100 μm) are sometimes included as part of the terahertz radiation range. Almost all black-body radiation from objects near room temperature is at infrared wavelengths. As a form of electromagnetic radiation, IR propagates energy and momentum, exerts radiation pressure, and has properties corresponding to both those of a wave and of a particle, the photon. It was long known that fires emit invisible heat; in 1681 the pioneering experimenter Edme Mariotte showed that glass, though transparent to sunlight, obstructed radiant heat. In 1800 the astronomer Sir William Herschel discovered ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Selenium
Selenium is a chemical element with the symbol Se and atomic number 34. It is a nonmetal (more rarely considered a metalloid) with properties that are intermediate between the elements above and below in the periodic table, sulfur and tellurium, and also has similarities to arsenic. It seldom occurs in its elemental state or as pure ore compounds in the Earth's crust. Selenium – from Greek ( 'Moon') – was discovered in 1817 by , who noted the similarity of the new element to the previously discovered tellurium (named for the Earth). Selenium is found in metal sulfide ores, where it partially replaces the sulfur. Commercially, selenium is produced as a byproduct in the refining of these ores, most often during production. Minerals that are pure selenide or selenate compounds are known but rare. The chief commercial uses for selenium today are glassmaking and pigments. Selenium is a semiconductor and is used in photocells. Applications in electronics, once important, have been ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Clausthalite
Clausthalite is a lead selenide mineral, PbSe. It forms a solid solution series with galena PbS. Occurrence It occurs in low-sulfur hydrothermal deposits with other selenides and in mercury deposits. It is associated with tiemannite, klockmannite, berzelianite, umangite, gold, stibiopalladinite and uraninite. It was first described in 1832 and named for the discovery locality of Clausthal-Zellerfeld in the Harz Mountains, Germany. See also * Classification of minerals * List of minerals This is a list of minerals for which there are articles on Wikipedia. Minerals are distinguished by various chemical and physical properties. Differences in chemical composition and crystal structure distinguish the various ''species''. Within a m ... References Lead minerals Selenide minerals Galena group {{mineral-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mineral
In geology and mineralogy, a mineral or mineral species is, broadly speaking, a solid chemical compound with a fairly well-defined chemical composition and a specific crystal structure that occurs naturally in pure form.John P. Rafferty, ed. (2011): Minerals'; p. 1. In the series ''Geology: Landforms, Minerals, and Rocks''. Rosen Publishing Group. The geological definition of mineral normally excludes compounds that occur only in living organisms. However, some minerals are often biogenic (such as calcite) or are organic compounds in the sense of chemistry (such as mellite). Moreover, living organisms often synthesize inorganic minerals (such as hydroxylapatite) that also occur in rocks. The concept of mineral is distinct from rock, which is any bulk solid geologic material that is relatively homogeneous at a large enough scale. A rock may consist of one type of mineral, or may be an aggregate of two or more different types of minerals, spacially segregated into distinct ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |