|
Laser-heated Pedestal Growth
Laser-heated pedestal growth (LHPG) or laser floating zone (LFZ) is a crystal growth technique. A narrow region of a crystal is melted with a powerful CO2 or YAG laser. The laser and hence the floating zone, is moved along the crystal. The molten region melts impure solid at its forward edge and leaves a wake of purer material solidified behind it. This technique for growing crystals from the melt (liquid/solid phase transition) is used in materials research. Advantages The main advantages of this technique are the high pulling rates (60 times greater than the conventional Czochralski technique) and the possibility of growing materials with very high melting points. In addition, LHPG is a crucible-free technique, which allows single crystals to be grown with high purity and low stress. The geometric shape of the crystals (the technique can produce small diameters), and the low production cost, make the single-crystal fibers (SCF) produced by LHPG suitable substitutes for bulk cr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Crystal Growth
A crystal is a solid material whose constituent atoms, molecules, or ions are arranged in an orderly repeating pattern extending in all three spatial dimensions. Crystal growth is a major stage of a crystallization process, and consists of the addition of new atoms, ions, or polymer strings into the characteristic arrangement of the crystalline lattice. The growth typically follows an initial stage of either homogeneous or heterogeneous (surface catalyzed) nucleation, unless a "seed" crystal, purposely added to start the growth, was already present. The action of crystal growth yields a crystalline solid whose atoms or molecules are close packed, with fixed positions in space relative to each other. The crystalline state of matter is characterized by a distinct structural rigidity and very high resistance to deformation (i.e. changes of shape and/or volume). Most crystalline solids have high values both of Young's modulus and of the shear modulus of elasticity. This c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
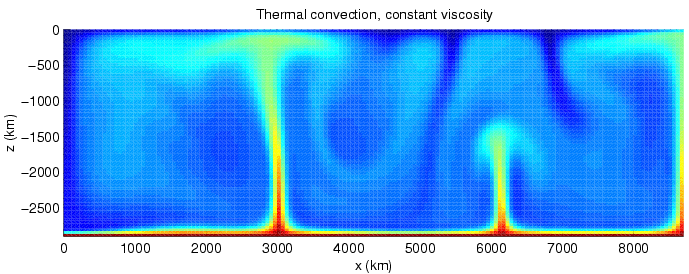
Convection
Convection is single or multiphase fluid flow that occurs spontaneously due to the combined effects of material property heterogeneity and body forces on a fluid, most commonly density and gravity (see buoyancy). When the cause of the convection is unspecified, convection due to the effects of thermal expansion and buoyancy can be assumed. Convection may also take place in soft solids or mixtures where particles can flow. Convective flow may be transient (such as when a multiphase mixture of oil and water separates) or steady state (see Convection cell). The convection may be due to gravitational, electromagnetic or fictitious body forces. Heat transfer by natural convection plays a role in the structure of Earth's atmosphere, its oceans, and its mantle. Discrete convective cells in the atmosphere can be identified by clouds, with stronger convection resulting in thunderstorms. Natural convection also plays a role in stellar physics. Convection is often cate ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Recrystallization (metallurgy)
In materials science, recrystallization is a process by which deformed grains are replaced by a new set of defect-free grains that nucleate and grow until the original grains have been entirely consumed. Recrystallization is usually accompanied by a reduction in the strength and hardness of a material and a simultaneous increase in the ductility.Thus, the process may be introduced as a deliberate step in metals processing or may be an undesirable byproduct of another processing step. The most important industrial uses are softening of metals previously hardened or rendered brittle by cold work, and control of the grain structure in the final product. Recrystallization temperature is typically 0.3–0.4 times the melting point for pure metals and 0.5 times for alloys. Definition Recrystallization is defined as the process in which grains of a crystal structure come in a new structure or new crystal shape. A precise definition of recrystallization is difficult to state as th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
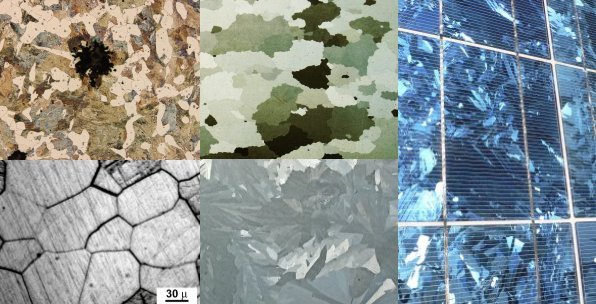
Protocrystalline
A protocrystalline phase is a distinct phase occurring during crystal growth which evolves into a microcrystalline form. The term is typically associated with silicon films in optical applications such as solar cells. Applications Silicon solar cells Amorphous silicon (a-Si) is a popular solar cell material owing to its low cost and ease of production. Owing to disordered structure ( Urbach tail), its absorption extends to the energies below the band gap resulting in a wide-range spectral response; however, it has a relatively low solar cell efficiency. Protocrystalline Si (pc-Si:H) also has a relatively low absorption near the band gap owing to its more ordered crystalline structure. Thus, protocrystalline and amorphous silicon can be combined in a tandem solar cell where the top thin layer of a-Si:H absorbs short-wavelength light whereas the longer wavelengths are absorbed by the underlying protocrystalline silicon layer. See also *Amorphous silicon *Crystallite *Multij ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nucleation
In thermodynamics, nucleation is the first step in the formation of either a new thermodynamic phase or structure via self-assembly or self-organization within a substance or mixture. Nucleation is typically defined to be the process that determines how long an observer has to wait before the new phase or self-organized structure appears. For example, if a volume of water is cooled (at atmospheric pressure) below 0°C, it will tend to freeze into ice, but volumes of water cooled only a few degrees below 0°C often stay completely free of ice for long periods (supercooling). At these conditions, nucleation of ice is either slow or does not occur at all. However, at lower temperatures nucleation is fast, and ice crystals appear after little or no delay. Nucleation is a common mechanism which generates first-order phase transitions, and it is the start of the process of forming a new thermodynamic phase. In contrast, new phases at continuous phase transitions start to form im ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Micro-pulling-down
The micro-pulling-down (μ-PD) method is a crystal growth technique based on continuous transport of the melted substance through micro-channel(s) made in a crucible bottom. Continuous solidification of the melt is progressed on a liquid/solid interface positioned under the crucible. In a steady state, both the melt and the crystal are pulled-down with a constant (but generally different) velocity. Many different types of crystal are grown by this technique, including Y3Al5O12, Si, Si-Ge, LiNbO3, α-Al2O3, Y2O3, Sc2O3, LiF, CaF2, BaF2, etc. Crystal growth routine Standard routine procedure used in the growth of most of μ-PD crystals is well developed. The general stages of the growths include: * Charging of the crucible with starting materials (mixture of powders) * Heating of the crucible until starting materials in the crucible are completely melted * Upward displacement of the seed until its contact with the meniscus or crucible * Formation of the meniscus and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fractional Crystallization (chemistry)
In chemistry, fractional crystallization is a method of refining substances based on differences in their solubility. It fractionates via differences in crystallization (forming of crystals). If a mixture of two or more substances in solution are allowed to crystallize, for example by allowing the temperature of the solution to decrease or increase, the precipitate will contain more of the least soluble substance. The proportion of components in the precipitate will depend on their solubility products. If the solubility products are very similar, a cascade process will be needed to effectuate a complete separation. This technique is often used in chemical engineering to obtain pure substances, or to recover saleable products from waste solutions. Fractional crystallization can be used to separate solid-solid mixtures. An example of this is separating KNO3 and KClO3.Viraf.J.Dalal Class 6/7 book See also * Cold Water Extraction * Fractional crystallization (geology) * ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Crystallization (engineering Aspects)
Crystallization is the process by which solid forms, where the atoms or molecules are highly organized into a structure known as a crystal. Some ways by which crystals form are precipitating from a solution, freezing, or more rarely deposition directly from a gas. Attributes of the resulting crystal depend largely on factors such as temperature, air pressure, and in the case of liquid crystals, time of fluid evaporation. Crystallization occurs in two major steps. The first is nucleation, the appearance of a crystalline phase from either a supercooled liquid or a supersaturated solvent. The second step is known as crystal growth, which is the increase in the size of particles and leads to a crystal state. An important feature of this step is that loose particles form layers at the crystal's surface and lodge themselves into open inconsistencies such as pores, cracks, etc. The majority of minerals and organic molecules crystallize easily, and the resulting crystals are g ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
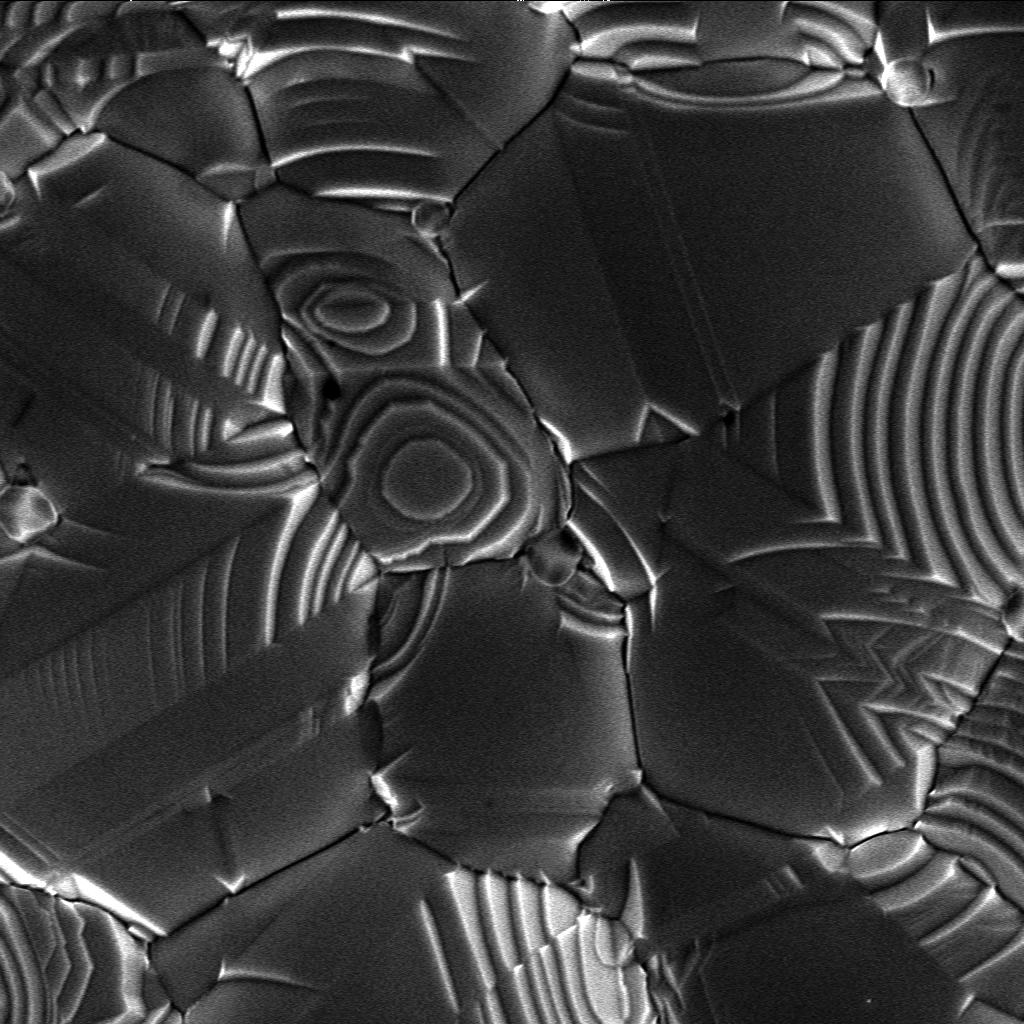
Crystallite
A crystallite is a small or even microscopic crystal which forms, for example, during the cooling of many materials. Crystallites are also referred to as grains. Bacillite is a type of crystallite. It is rodlike with parallel longulites. Structure The orientation of crystallites can be random with no preferred direction, called random texture, or directed, possibly due to growth and processing conditions. While the structure of a ( single) crystal is highly ordered and its lattice is continuous and unbroken, amorphous materials, such as glass and many polymers, are non-crystalline and do not display any structures, as their constituents are not arranged in an ordered manner. Polycrystalline structures and paracrystalline phases are in-between these two extremes. Polycrystalline materials, or polycrystals, are solids that are composed of many crystallites of varying size and orientation. Most materials are polycrystalline, made of a large number crystallites held together by ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Crystal Structure
In crystallography, crystal structure is a description of the ordered arrangement of atoms, ions or molecules in a crystalline material. Ordered structures occur from the intrinsic nature of the constituent particles to form symmetric patterns that repeat along the principal directions of three-dimensional space in matter. The smallest group of particles in the material that constitutes this repeating pattern is the unit cell of the structure. The unit cell completely reflects the symmetry and structure of the entire crystal, which is built up by repetitive translation of the unit cell along its principal axes. The translation vectors define the nodes of the Bravais lattice. The lengths of the principal axes, or edges, of the unit cell and the angles between them are the lattice constants, also called ''lattice parameters'' or ''cell parameters''. The symmetry properties of the crystal are described by the concept of space groups. All possible symmetric arrangements of partic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Platinum
Platinum is a chemical element with the symbol Pt and atomic number 78. It is a dense, malleable, ductile, highly unreactive, precious, silverish-white transition metal. Its name originates from Spanish , a diminutive of "silver". Platinum is a member of the platinum group of elements and group 10 of the periodic table of elements. It has six naturally occurring isotopes. It is one of the rarer elements in Earth's crust, with an average abundance of approximately 5 μg/kg. It occurs in some nickel and copper ores along with some native deposits, mostly in South Africa, which accounts for ~80% of the world production. Because of its scarcity in Earth's crust, only a few hundred tonnes are produced annually, and given its important uses, it is highly valuable and is a major precious metal commodity. Platinum is one of the least reactive metals. It has remarkable resistance to corrosion, even at high temperatures, and is therefore considered a noble metal. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |