|
Lanthanide Trichloride
Lanthanide trichlorides are a family of inorganic compound with the formula Ln Cl3, where Ln stands for a lanthanide metal. The trichlorides are standard reagents in applied and academic chemistry of the lanthanides. They exist as anhydrous solids and as hydrates. Properties The anhydrous solids have melting points range from ca. 582 (Tb) - 925 °C (Lu). They are generally pale colored, often white. As coordination polymers, they only dissolve in donor solvents, including water. Preparation The lanthanide oxides and carbonates dissolve in hydrochloric acid to give chloride salt of the hydrated cations: :M2O3 + 6HCl + n H2O → 2 n(H2O)nl3 Industrial routes Anhydrous trichlorides are produced commercially by carbothermic reaction of the oxide: :M2O3 + 3Cl2 + 3C → 2MCl3 + 3CO Ammonium chloride route The ammonium chloride route refers to a general procedure to produce anhydrous lanthanide chlorides. The method has the advantages of being general for the 14 la ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Inorganic Compound
In chemistry, an inorganic compound is typically a chemical compound that lacks carbon–hydrogen bonds, that is, a compound that is not an organic compound. The study of inorganic compounds is a subfield of chemistry known as '' inorganic chemistry''. Inorganic compounds comprise most of the Earth's crust, although the compositions of the deep mantle remain active areas of investigation. Some simple carbon compounds are often considered inorganic. Examples include the allotropes of carbon (graphite, diamond, buckminsterfullerene, etc.), carbon monoxide, carbon dioxide, carbides, and the following salts of inorganic anions: carbonates, cyanides, cyanates, and thiocyanates. Many of these are normal parts of mostly organic systems, including organisms; describing a chemical as inorganic does not necessarily mean that it does not occur within living things. History Friedrich Wöhler's conversion of ammonium cyanate into urea in 1828 is often cited as the starting point of modern ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
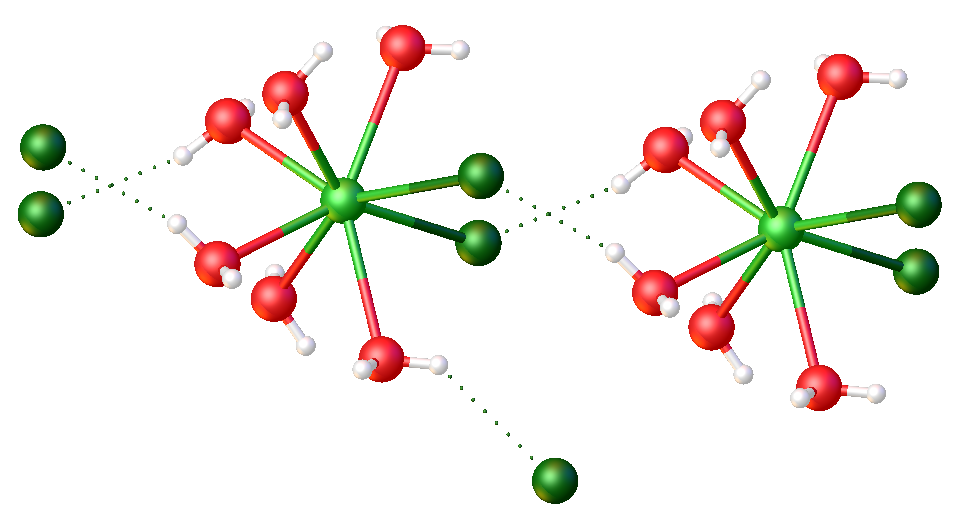
Thulium Trichloride
Thulium(III) chloride or thulium trichloride is as an inorganic salt composed of thulium and chlorine with the formula In science, a formula is a concise way of expressing information symbolically, as in a mathematical formula or a ''chemical formula''. The informal use of the term ''formula'' in science refers to the general construct of a relationship betwee ... TmCl3. It forms yellow crystals. Thulium(III) chloride has the YCl3 ( AlCl3) layer structure with octahedral thulium ions.Wells A.F. (1984) ''Structural Inorganic Chemistry'' 5th edition Oxford Science Publications Reactions The hydrated form of thulium(III) chloride can be obtained by adding thulium(III) oxide to concentrated hydrochloric acid. Thulium(III) chloride reacts with strong bases to make thulium(III) oxide. References Thulium compounds Chlorides Lanthanide halides {{inorganic-compound-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrogen Chloride
The compound hydrogen chloride has the chemical formula and as such is a hydrogen halide. At room temperature, it is a colourless gas, which forms white fumes of hydrochloric acid upon contact with atmospheric water vapor. Hydrogen chloride gas and hydrochloric acid are important in technology and industry. Hydrochloric acid, the aqueous solution of hydrogen chloride, is also commonly given the formula HCl. Reactions Hydrogen chloride is a diatomic molecule, consisting of a hydrogen atom H and a chlorine atom Cl connected by a polar covalent bond. The chlorine atom is much more electronegative than the hydrogen atom, which makes this bond polar. Consequently, the molecule has a large dipole moment with a negative partial charge (δ−) at the chlorine atom and a positive partial charge (δ+) at the hydrogen atom. In part because of its high polarity, HCl is very soluble in water (and in other polar solvents). Upon contact, and HCl combine to form hydronium cations and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ytterbium Oxide
Ytterbium(III) oxide is the chemical compound with the formula Yb2O3. It is one of the more commonly encountered compounds of ytterbium. It has the "rare-earth C-type sesquioxide" structure which is related to the fluorite structure with one quarter of the anions removed, leading to ytterbium atoms in two different six coordinate (non-octahedral) environments. Wells A.F. (1984) ''Structural Inorganic Chemistry'' 5th edition Oxford Science Publications Uses * Colorant for glasses and enamels * Dopant for garnet crystals in lasers * Optical fibers See also * Active laser medium The active laser medium (also called gain medium or lasing medium) is the source of optical gain within a laser. The gain results from the stimulated emission of photons through electronic or molecular transitions to a lower energy state from a h ... References External links Information at WebElements Ytterbium compounds Sesquioxides {{inorganic-compound-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lutetium Oxide
Lutetium(III) oxide, a white solid, is a cubic compound of lutetium sometimes used in the preparation of specialty glasses. It is also called lutecia. It is a lanthanide oxide, also known as a rare earth.Lutetium Oxide. 1997-2007. Metall Rare Earth Limited. http://www.metall.com.cn/luo.htm History In 1879, Jean Charles Galissard de Marignac (1817–1894), a Swiss chemist, claimed to have discovered ytterbium, but he had found a mixture of elements. In 1907, a French chemist Georges Urbain (1872–1938) reported that ytterbium was a mixture of two new elements and was not a single element. Two other chemists, Carl Auer von Welsbach (1858–1929) and Charles James (1880–1926) also extracted lutetium(III) oxide around the same time. All three scientists successfully separated Marignac's ytterbia into oxides of two elements which were eventually named ytterbium and lutetium Lutetium is a chemical element with the symbol Lu and atomic number 71. It is a silvery white metal, which ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thulium(III) Oxide
Thulium(III) oxide is a pale green solid compound, with the formula Tm2 O3. It was first isolated in 1879, from an impure sample of erbia, by Swedish chemist Per Teodor Cleve, who named it ''thulia''. It can be prepared in the laboratory by burning thulium Thulium is a chemical element with the symbol Tm and atomic number 69. It is the thirteenth and third-last element in the lanthanide series. Like the other lanthanides, the most common oxidation state is +3, seen in its oxide, halides and other c ... metal in air, or by decomposition of their oxoacid salts, such as thulium nitrate. References {{Oxides Thulium compounds Sesquioxides ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Erbium(III) Oxide
Erbium(III) oxide is the inorganic compound with the formula . It is a pink paramagnetic solid. It finds uses in various optical materials. Structure Erbium(III) oxide has a cubic structure resembling the bixbyite motif. The Er3+ centers are octahedral. Reactions The formation of erbium oxide is accomplished by burning erbium metal. Erbium oxide is insoluble in water and soluble in mineral acids. Er2O3 does not readily absorb moisture and carbon dioxide from the atmosphere. It can react with acids to form the corresponding erbium(III) salts. For example, with hydrochloric acid, the oxide follows the following idealized reaction leading to erbium chloride: : In practice, such simple acid-base reactions are accompanied by hydration: : Properties One interesting property of erbium oxides is their ability to up convert photons. Photon upconversion takes place when infrared or visible radiation, low energy light, is converted to ultraviolet or violet radiation higher energy l ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Holmium Oxide
Holmium(III) oxide, or holmium oxide is a chemical compound of a rare-earth element holmium and oxygen with the formula Ho2O3. Together with dysprosium(III) oxide (Dy2O3), holmium oxide is one of the most powerfully paramagnetic substances known. The oxide, also called holmia, occurs as a component of the related erbium oxide mineral called erbia. Typically, the oxides of the trivalent lanthanides coexist in nature, and separation of these components requires specialized methods. Holmium oxide is used in making specialty colored glasses. Glass containing holmium oxide and holmium oxide solutions have a series of sharp optical absorption peaks in the visible spectral range. They are therefore traditionally used as a convenient calibration standard for optical spectrophotometers. Properties Appearance Holmium oxide has some fairly dramatic color changes depending on the lighting conditions. In daylight, it is a tannish yellow color. Under trichromatic light, it is a fiery orange ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dysprosium Oxide
Dysprosium oxide (Dy2O3) is a sesquioxide compound of the rare earth metal dysprosium. It is a pastel yellowish-greenish, slightly hygroscopic powder having specialized uses in ceramics, glass, phosphors, lasers, as a Faraday rotator and dysprosium metal halide lamp A metal (from Greek μέταλλον ''métallon'', "mine, quarry, metal") is a material that, when freshly prepared, polished, or fractured, shows a lustrous appearance, and conducts electricity and heat relatively well. Metals are typicall ...s. It can react with acids to produce the corresponding dysprosium(III) salts: :Dy2O3 + 6 HCl → 2 DyCl3 + 3 H2O References Dysprosium compounds Sesquioxides {{Inorganic-compound-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Yttrium
Yttrium is a chemical element with the symbol Y and atomic number 39. It is a silvery-metallic transition metal chemically similar to the lanthanides and has often been classified as a "rare-earth element". Yttrium is almost always found in combination with lanthanide elements in rare-earth minerals, and is never found in nature as a free element. 89Y is the only stable isotope, and the only isotope found in the Earth's crust. The most important uses of yttrium are LEDs and phosphors, particularly the red phosphors in television set cathode ray tube displays. Yttrium is also used in the production of electrodes, electrolytes, electronic filters, lasers, superconductors, various medical applications, and tracing various materials to enhance their properties. Yttrium has no known biological role. Exposure to yttrium compounds can cause lung disease in humans. The element is named after '' ytterbite'', a mineral first identified in 1787 by the chemist Carl Axel Arrhenius. He n ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Scandium
Scandium is a chemical element with the symbol Sc and atomic number 21. It is a silvery-white metallic d-block element. Historically, it has been classified as a rare-earth element, together with yttrium and the Lanthanides. It was discovered in 1879 by spectral analysis of the minerals euxenite and gadolinite from Scandinavia. Scandium is present in most of the deposits of rare-earth and uranium compounds, but it is extracted from these ores in only a few mines worldwide. Because of the low availability and difficulties in the preparation of metallic scandium, which was first done in 1937, applications for scandium were not developed until the 1970s, when the positive effects of scandium on aluminium alloys were discovered, and its use in such alloys remains its only major application. The global trade of scandium oxide is 15–20 tonnes per year. The properties of scandium compounds are intermediate between those of aluminium and yttrium. A diagonal relationship exists betwee ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Anhydrous
A substance is anhydrous if it contains no water. Many processes in chemistry can be impeded by the presence of water; therefore, it is important that water-free reagents and techniques are used. In practice, however, it is very difficult to achieve perfect dryness; anhydrous compounds gradually absorb water from the atmosphere so they must be stored carefully. Solids Many salts and solids can be dried using heat, or under vacuum. Desiccators can also be used to store reagents in dry conditions. Common desiccants include phosphorus pentoxide and silica gel. Chemists may also require dry glassware for sensitive reactions. This can be achieved by drying glassware in an oven, by flame, or under vacuum. Dry solids can be produced by freeze-drying, which is also known as lyophilization. Liquids or solvents In many cases, the presence of water can prevent a reaction from happening, or cause undesirable products to form. To prevent this, anhydrous solvents must be used when performi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |