|
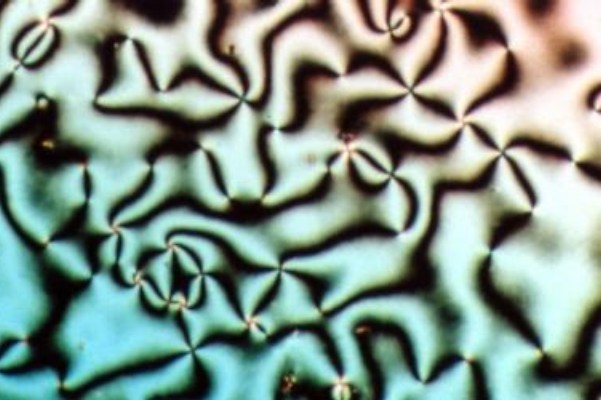
Lamellar Phase
Lamellar phase refers generally to packing of polar-headed long chain nonpolar-tail molecules in an environment of bulk polar liquid, as sheets of bilayers separated by bulk liquid. In biophysics, polar lipids (mostly, phospholipids, and rarely, glycolipids) pack as a liquid crystalline bilayer, with hydrophobic fatty acyl long chains directed inwardly and polar headgroups of lipids aligned on the outside in contact with water, as a 2-dimensional flat sheet surface. Under transmission electron microscope (TEM), after staining with polar headgroup reactive chemical osmium tetroxide, lamellar lipid phase appears as two thin parallel dark staining lines/sheets, constituted by aligned polar headgroups of lipids. 'Sandwiched' between these two parallel lines, there exists one thicker line/sheet of non-staining closely packed layer of long lipid fatty acyl chains. This TEM-appearance became famous as Robertson's unit membrane - the basis of all biological membranes, and structure of lipid ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Polarity
In chemistry, polarity is a separation of electric charge leading to a molecule or its chemical groups having an electric dipole moment, with a negatively charged end and a positively charged end. Polar molecules must contain one or more polar bonds due to a difference in electronegativity between the bonded atoms. Molecules containing polar bonds have no molecular polarity if the bond dipoles cancel each other out by symmetry. Polar molecules interact through dipole–dipole intermolecular forces and hydrogen bonds. Polarity underlies a number of physical properties including surface tension, solubility, and melting and boiling points. Polarity of bonds Not all atoms attract electrons with the same force. The amount of "pull" an atom exerts on its electrons is called its electronegativity. Atoms with high electronegativitiessuch as fluorine, oxygen, and nitrogenexert a greater pull on electrons than atoms with lower electronegativities such as alkali metals and alkaline ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fatty-acid
In chemistry, particularly in biochemistry, a fatty acid is a carboxylic acid with an aliphatic chain, which is either saturated or unsaturated. Most naturally occurring fatty acids have an unbranched chain of an even number of carbon atoms, from 4 to 28. Fatty acids are a major component of the lipids (up to 70% by weight) in some species such as microalgae but in some other organisms are not found in their standalone form, but instead exist as three main classes of esters: triglycerides, phospholipids, and cholesteryl esters. In any of these forms, fatty acids are both important dietary sources of fuel for animals and important structural components for cells. History The concept of fatty acid (''acide gras'') was introduced in 1813 by Michel Eugène Chevreul, though he initially used some variant terms: ''graisse acide'' and ''acide huileux'' ("acid fat" and "oily acid"). Types of fatty acids Fatty acids are classified in many ways: by length, by saturation vs unsaturation, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Surfactants
Surfactants are chemical compounds that decrease the surface tension between two liquids, between a gas and a liquid, or interfacial tension between a liquid and a solid. Surfactants may act as detergents, wetting agents, emulsion#Emulsifiers , emulsifiers, foaming agents, or dispersants. The word "surfactant" is a Blend word, blend of ''surface-active agent'', coined . Agents that increase surface tension are "surface active" in the literal sense but are not called surfactants as their effect is opposite to the common meaning. A common example of surface tension increase is salting out: by adding an inorganic salt to an aqueous solution of a weakly polar substance, the substance will precipitate. The substance may itself be a surfactant – this is one of the reasons why many surfactants are ineffective in sea water. Composition and structure Surfactants are usually organic compounds that are amphiphilic, meaning each molecule contains both a hydrophilic "water-seeking" ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Membrane Biology
Membrane biology is the study of the biological and physiochemical characteristics of membranes, with applications in the study of cellular physiology. Membrane bioelectrical impulses are described by the Hodgkin cycle. Biophysics Membrane biophysics is the study of biological membrane structure and function using physical, computational, mathematical, and biophysical methods. A combination of these methods can be used to create phase diagrams of different types of membranes, which yields information on thermodynamic behavior of a membrane and its components. As opposed to membrane biology, membrane biophysics focuses on quantitative information and modeling of various membrane phenomena, such as lipid raft The plasma membranes of cells contain combinations of glycosphingolipids, cholesterol and protein receptors organised in glycolipoprotein lipid microdomains termed lipid rafts. Their existence in cellular membranes remains somewhat controversial. ... formation, rates of l ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Liquid Crystals
Liquid crystal (LC) is a state of matter whose properties are between those of conventional liquids and those of solid crystals. For example, a liquid crystal may flow like a liquid, but its molecules may be oriented in a crystal-like way. There are many types of LC phases, which can be distinguished by their optical properties (such as textures). The contrasting textures arise due to molecules within one area of material ("domain") being oriented in the same direction but different areas having different orientations. LC materials may not always be in a LC state of matter (just as water may be ice or water vapor). Liquid crystals can be divided into 3 main types: * thermotropic, *lyotropic, and * metallotropic. Thermotropic and lyotropic liquid crystals consist mostly of organic molecules, although a few minerals are also known. Thermotropic LCs exhibit a phase transition into the LC phase as temperature changes. Lyotropic LCs exhibit phase transitions as a function of bo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Unilamellar Liposome
A unilamellar liposome is a spherical liposome, a vesicle, bounded by a single bilayer of an amphiphilic lipid or a mixture of such lipids, containing aqueous solution inside the chamber. Unilamellar liposomes are used to study biological systems and to mimic cell membranes, and are classified into three groups based on their size: small unilamellar liposomes/vesicles (SUVs) that with a size range of 20–100 nm, large unilamellar liposomes/vesicles (LUVs) with a size range of 100–1000 nm and giant unilamellar liposomes/vesicles (GUVs) with a size range of 1-200 µm. GUVs are mostly used as models for biological membranes in research work. Animal cells are 10–30 µm and plant cells are typically 10–100 µm. Even smaller cell organelles such as mitochondria are typically 1-2 µm. Therefore, a proper model should account for the size of the specimen being studied. In addition, the size of vesicles dictates their membrane curvature which is an import ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Micelle
A micelle () or micella () (plural micelles or micellae, respectively) is an aggregate (or supramolecular assembly) of surfactant amphipathic lipid molecules dispersed in a liquid, forming a colloidal suspension (also known as associated colloidal system). A typical micelle in water forms an aggregate with the hydrophilic "head" regions in contact with surrounding solvent, sequestering the hydrophobic single-tail regions in the micelle centre. This phase is caused by the packing behavior of single-tail lipids in a bilayer. The difficulty filling all the volume of the interior of a bilayer, while accommodating the area per head group forced on the molecule by the hydration of the lipid head group, leads to the formation of the micelle. This type of micelle is known as a normal-phase micelle (oil-in-water micelle). Inverse micelles have the head groups at the centre with the tails extending out (water-in-oil micelle). Micelles are approximately spherical in shape. Other phases ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lipid Bilayer
The lipid bilayer (or phospholipid bilayer) is a thin polar membrane made of two layers of lipid molecules. These membranes are flat sheets that form a continuous barrier around all cells. The cell membranes of almost all organisms and many viruses are made of a lipid bilayer, as are the nuclear membrane surrounding the cell nucleus, and membranes of the membrane-bound organelles in the cell. The lipid bilayer is the barrier that keeps ions, proteins and other molecules where they are needed and prevents them from diffusing into areas where they should not be. Lipid bilayers are ideally suited to this role, even though they are only a few nanometers in width, because they are impermeable to most water-soluble (hydrophilic) molecules. Bilayers are particularly impermeable to ions, which allows cells to regulate salt concentrations and pH by transporting ions across their membranes using proteins called ion pumps. Biological bilayers are usually composed of amphiphilic phosphol ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lipid Polymorphism
Polymorphism in biophysics is the ability of lipids to aggregate in a variety of ways, giving rise to structures of different shapes, known as "phases". This can be in the form of sphere of lipid molecules (micelles), pairs of layers that face one another (lamellar phase, observed in biological systems as a lipid bilayer), a tubular arrangement (hexagonal), or various cubic phases (Fdm, Imm, Iam, Pnm, and Pmm being those discovered so far). More complicated aggregations have also been observed, such as rhombohedral, tetragonal and orthorhombic phases. It forms an important part of current academic research in the fields of membrane biophysics (polymorphism), biochemistry (biological impact) and organic chemistry (synthesis). Determination of the topology of a lipid system is possible by a number of methods, the most reliable of which is x-ray diffraction. This uses a beam of x-rays that are scattered by the sample, giving a diffraction pattern as a set of rings. The ratio of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
History Of Cell Membrane Theory
Cell theory has its origins in seventeenth century histology, microscopy observations, but it was nearly two hundred years before a complete cell membrane theory was developed to explain what separates cells from the outside world. By the 19th century it was accepted that some form of semi-permeable barrier must exist around a cell. Studies of the action of anesthetic molecules led to the theory that this barrier might be made of some sort of fat (lipid), but the structure was still unknown. A series of pioneering experiments in 1925 indicated that this barrier membrane consisted of two molecular layers of lipids—a lipid bilayer. New tools over the next few decades confirmed this theory, but controversy remained regarding the role of proteins in the cell membrane. Eventually the Cell membrane#Structure and the Fluid mosaic model, fluid mosaic model was composed in which proteins “float” in a fluid lipid bilayer "sea". Although simplistic and incomplete, this model is still widel ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lubricant
A lubricant (sometimes shortened to lube) is a substance that helps to reduce friction between surfaces in mutual contact, which ultimately reduces the heat generated when the surfaces move. It may also have the function of transmitting forces, transporting foreign particles, or heating or cooling the surfaces. The property of reducing friction is known as lubricity. In addition to industrial applications, lubricants are used for many other purposes. Other uses include cooking (oils and fats in use in frying pans, in baking to prevent food sticking), bioapplications on humans (e.g. lubricants for artificial joints), ultrasound examination, medical examination, and sexual intercourse. It is mainly used to reduce friction and to contribute to a better and efficient functioning of a mechanism. History Lubricants have been in some use for thousands of years. Calcium soaps have been identified on the axles of chariots dated to 1400 BC. Building stones were slid on oil-impregrated lumb ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Spectroscopy
Spectroscopy is the field of study that measures and interprets the electromagnetic spectra that result from the interaction between electromagnetic radiation and matter as a function of the wavelength or frequency of the radiation. Matter waves and acoustic waves can also be considered forms of radiative energy, and recently gravitational waves have been associated with a spectral signature in the context of the Laser Interferometer Gravitational-Wave Observatory (LIGO) In simpler terms, spectroscopy is the precise study of color as generalized from visible light to all bands of the electromagnetic spectrum. Historically, spectroscopy originated as the study of the wavelength dependence of the absorption by gas phase matter of visible light dispersed by a prism. Spectroscopy, primarily in the electromagnetic spectrum, is a fundamental exploratory tool in the fields of astronomy, chemistry, materials science, and physics, allowing the composition, physical structure and e ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |