|
Kumada-Corriu Coupling
In organic chemistry, the Kumada coupling is a type of cross coupling reaction, useful for generating carbon–carbon bonds by the reaction of a Grignard reagent and an organic halide. The procedure uses transition metal catalysts, typically nickel or palladium, to couple a combination of two alkyl, aryl or vinyl groups. The groups of Robert Corriu and Makoto Kumada reported the reaction independently in 1972. The reaction is notable for being among the first reported catalytic cross-coupling methods. Despite the subsequent development of alternative reactions (Suzuki, Sonogashira, Stille, Hiyama, Negishi), the Kumada coupling continues to be employed in many synthetic applications, including the industrial-scale production of aliskiren, a hypertension medication, and polythiophenes, useful in organic electronic devices. History The first investigations into the catalytic coupling of Grignard reagents with organic halides date back to the 1941 study of cobalt catalysts ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Makoto Kumada
was a Japanese chemist and was a Professor of Chemistry first at Osaka City University until his retirement in 1983 at Kyoto University in Japan. In 1972, Kumada's group reported nickel-catalyzed cross coupling reactions nearly concurrently with the Corriu group working in France. The Kumada coupling In organic chemistry, the Kumada coupling is a type of cross coupling reaction, useful for generating carbon–carbon bonds by the reaction of a Grignard reagent and an organic halide. The procedure uses transition metal catalysts, typically ... now bears his name. References * 1920 births 2007 deaths Japanese chemists People from Fukui Prefecture Kyoto University alumni Kyoto University faculty {{chemist-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Synthesis
As a topic of chemistry, chemical synthesis (or combination) is the artificial execution of chemical reactions to obtain one or several products. This occurs by physical and chemical manipulations usually involving one or more reactions. In modern laboratory uses, the process is reproducible and reliable. A chemical synthesis involves one or more compounds (known as '' reagents'' or ''reactants'') that will experience a transformation when subjected to certain conditions. Various reaction types can be applied to formulate a desired product. This requires mixing the compounds in a reaction vessel, such as a chemical reactor or a simple round-bottom flask. Many reactions require some form of processing (" work-up") or purification procedure to isolate the final product. The amount produced by chemical synthesis is known as the ''reaction yield''. Typically, yields are expressed as a mass in grams (in a laboratory setting) or as a percentage of the total theoretical quantity that ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tosylate
In organic chemistry, a toluenesulfonyl group (tosyl group, abbreviated Ts or Tos) is a univalent functional group with the chemical formula –. It consists of a tolyl group, –, joined to a sulfonyl group, ––, with the open valence on sulfur. This group is usually derived from the compound tosyl chloride, (abbreviated TsCl), which forms esters and amides of toluenesulfonic acid, (abbreviated TsOH). The para orientation illustrated (''p''-toluenesulfonyl) is most common, and by convention ''tosyl'' without a prefix refers to the ''p''-toluenesulfonyl group. The toluenesulfonate (or tosylate) group refers to the – (TsO–) group, with an additional oxygen attached to sulfur and open valence on an oxygen. In a chemical name, the term ''tosylate'' may either refer to the salts containing the anion of ''p''-toluenesulfonic acid, (M = alkali metal, , , etc), or it may refer to esters of ''p''-toluenesulfonic acid, TsOR (R = organyl group). Applications For SN2 reacti ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
E3 Ciclo Catalitico Nichel
E3 (short for Electronic Entertainment Expo or Electronic Entertainment Experience in 2021) is a trade event for the video game industry. The Entertainment Software Association (ESA) organizes and presents E3, which many developers, publishers, hardware, and accessory manufacturers use to introduce and advertise upcoming games and game-related merchandise to retailers and to members of the press. E3 includes an exhibition floor for developers, publishers, and manufacturers to showcase their titles and products for sale in the upcoming year. Before and during the event, publishers and hardware manufacturers usually hold press conferences to announce new games and products. Over time, E3 has been considered the largest gaming-expo of the year by importance and impact. Before 2017, E3 was an industry-only event; the ESA required individuals wishing to attend to verify a professional relationship with the video game industry. With the rise of streaming media, several of the press ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oxidative Addition
Oxidative addition and reductive elimination are two important and related classes of reactions in organometallic chemistry. Oxidative addition is a process that increases both the oxidation state and coordination number of a metal centre. Oxidative addition is often a step in catalytic cycles, in conjunction with its reverse reaction, reductive elimination. Role in transition metal chemistry For transition metals, oxidative reaction results in the decrease in the d''n'' to a configuration with fewer electrons, often 2e fewer. Oxidative addition is favored for metals that are (i) basic and/or (ii) easily oxidized. Metals with a relatively low oxidation state often satisfy one of these requirements, but even high oxidation state metals undergo oxidative addition, as illustrated by the oxidation of Pt(II) with chlorine: : tCl4sup>2− + Cl2 → tCl6sup>2− In classical organometallic chemistry, the formal oxidation state of the metal and the electron count of the complex both in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
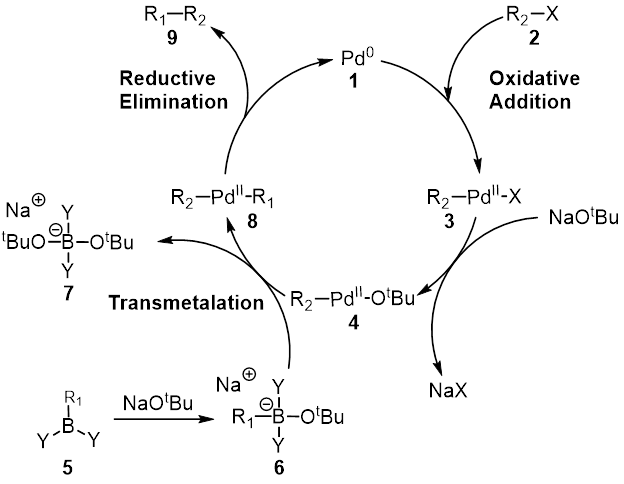
Kumada Catalytic Cycle
Kumada (written: lit. "bear rice field") is a Japanese surname. Notable people with the surname include: *, Japanese chemist *, Japanese child actress *, Japanese gravure idol and singer *, Japanese footballer and manager See also *Kumada coupling In organic chemistry, the Kumada coupling is a type of cross coupling reaction, useful for generating carbon–carbon bonds by the reaction of a Grignard reagent and an organic halide. The procedure uses transition metal catalysts, typically n ..., a cross coupling reaction {{surname Japanese-language surnames ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Richard F
Richard is a male given name. It originates, via Old French, from Old Frankish and is a compound of the words descending from Proto-Germanic ''*rīk-'' 'ruler, leader, king' and ''*hardu-'' 'strong, brave, hardy', and it therefore means 'strong in rule'. Nicknames include "Richie", "Dick", "Dickon", " Dickie", "Rich", "Rick", "Rico", "Ricky", and more. Richard is a common English, German and French male name. It's also used in many more languages, particularly Germanic, such as Norwegian, Danish, Swedish, Icelandic, and Dutch, as well as other languages including Irish, Scottish, Welsh and Finnish. Richard is cognate with variants of the name in other European languages, such as the Swedish "Rickard", the Catalan "Ricard" and the Italian "Riccardo", among others (see comprehensive variant list below). People named Richard Multiple people with the same name * Richard Andersen (other) * Richard Anderson (other) * Richard Cartwright (other) * Ri ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Akira Suzuki (chemist)
is a Japanese chemist and Nobel Prize Laureate (2010), who first published the Suzuki reaction, the organic reaction of an aryl- or vinyl-boronic acid with an aryl- or vinyl- halide catalyzed by a palladium(0) complex, in 1979. Early life and education Suzuki was born on September 12, 1930, in Mukawa, Hokkaidō, his father died when he was in high school. He studied chemistry at Hokkaido University (Hokudai) and after receiving his PhD while he worked there as assistant professor. He initially wanted to major in mathematics, his favorite subject in childhood was arithmetic.『朝日小学生新聞』2010年10月8日 It was an encounter with two books that became an opportunity to advance to the path of organic synthesis, one is ''Textbook of Organic Chemistry'' written by Louis Fieser of Harvard University, and another is ''Hydroboration'' written by Herbert C. Brown of Purdue University. Career From 1963 until 1965, Suzuki worked as a postdoctoral student with Herbert ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ei-ichi Negishi
was a Japanese chemist who was best known for his discovery of the Negishi coupling. He spent most of his career at Purdue University in the United States, where he was the Herbert C. Brown Distinguished Professor and the director of the Negishi-Brown Institute. He was awarded the 2010 Nobel Prize in Chemistry "for palladium catalyzed cross couplings in organic synthesis" jointly with Richard F. Heck and Akira Suzuki.Press releaseGreat art in a test tube Royal Swedish Academy of Sciences. Accessed October 6, 2010. Early life and education Negishi was born in Hsinking (today known as Changchun), the capital of Manchukuo, in July 1935. Following the transfer of his father who worked at the South Manchuria Railway in 1936, he moved to Harbin, and lived eight years there. In 1943, when he was nine, the Negishi family moved to Incheon, and a year later to Kyongsong Prefecture (now Seoul), both in Japanese-occupied Korea. In November 1945, three months after World War II ended, t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nobel Prize In Chemistry
) , image = Nobel Prize.png , alt = A golden medallion with an embossed image of a bearded man facing left in profile. To the left of the man is the text "ALFR•" then "NOBEL", and on the right, the text (smaller) "NAT•" then "MDCCCXXXIII" above, followed by (smaller) "OB•" then "MDCCCXCVI" below. , awarded_for = Outstanding contributions in chemistry , presenter = Royal Swedish Academy of Sciences , location = Stockholm, Sweden , reward = 9 million SEK (2017) , year = 1901 , holder = Carolyn R. Bertozzi, Morten P. Meldal and Karl Barry Sharpless (2022) , most_awards = Frederick Sanger and Karl Barry Sharpless (2) , website nobelprize.org, previous = 2021 , year2=2022, main=2022, next=2023 The Nobel Prize in Chemistry is awarded annually by the Royal Swedish Academy of Sciences to scientists in the various fields of chemistry. It is one of the five Nobel Prizes established by the will of Alfred Nobel in 1895, awarded for ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Morris S
Morris may refer to: Places Australia *St Morris, South Australia, place in South Australia Canada * Morris Township, Ontario, now part of the municipality of Morris-Turnberry * Rural Municipality of Morris, Manitoba ** Morris, Manitoba, a town mostly surrounded by the municipality * Morris (electoral district), Manitoba (defunct) * Rural Municipality of Morris No. 312, Saskatchewan United States ;Communities * Morris, Alabama, a town * Morris, Connecticut, a town * Morris, Georgia, an unincorporated community * Morris, Illinois, a city * Morris, Indiana, an unincorporated community * Morris, Minnesota, a city * Morristown, New Jersey, a town * Morris (town), New York ** Morris (village), New York * Morris, Oklahoma, a city * Morris, Pennsylvania, an unincorporated community * Morris, West Virginia, an unincorporated community * Morris, Kanawha County, West Virginia, a ghost town * Morris, Wisconsin, a town * Morris Township (other) ;Counties and other ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cobalt
Cobalt is a chemical element with the symbol Co and atomic number 27. As with nickel, cobalt is found in the Earth's crust only in a chemically combined form, save for small deposits found in alloys of natural meteoric iron. The free element, produced by reductive smelting, is a hard, lustrous, silver-gray metal. Cobalt-based blue pigments ( cobalt blue) have been used since ancient times for jewelry and paints, and to impart a distinctive blue tint to glass, but the color was for a long time thought to be due to the known metal bismuth. Miners had long used the name ''kobold ore'' (German for ''goblin ore'') for some of the blue-pigment-producing minerals; they were so named because they were poor in known metals, and gave poisonous arsenic-containing fumes when smelted. In 1735, such ores were found to be reducible to a new metal (the first discovered since ancient times), and this was ultimately named for the ''kobold''. Today, some cobalt is produced specifically from one of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |