|
Klimisch Score
The Klimisch score is a method of assessing the reliability of toxicological studies, mainly for regulatory purposes, that was proposed by H.J. Klimisch, M. Andreae and U. Tillmann of the chemical company BASF in 1997 in a paper entitled ''A Systematic Approach for Evaluating the Quality of Experimental Toxicological and Ecotoxicological Data'' which was published in ''Regulatory Toxicology and Pharmacology''. It assigns studies to one of four categories as follows: The applicable guidelines are the (OECD Guidelines for the Testing of Chemicals, EU Test Methods), and other such methods. Often studies are performed to more than one test guideline where they are in agreement as to the requirements. GLP is Good Laboratory Practice. The scoring system is the standard method used in both the EU regulatory schemes (e.g. REACH Regulation). Generally, only Klimisch scores of 1 or 2 can be used by themselves to cover an endpoint. However, Klimisch score 3 and 4 data can still be used a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Toxicological
Toxicology is a scientific discipline, overlapping with biology, chemistry, pharmacology, and medicine, that involves the study of the adverse effects of chemical substances on living organisms and the practice of diagnosing and treating exposures to toxins and toxicants. The relationship between dose and its effects on the exposed organism is of high significance in toxicology. Factors that influence chemical toxicity include the dosage, duration of exposure (whether it is acute or chronic), route of exposure, species, age, sex, and environment. Toxicologists are experts on poisons and poisoning. There is a movement for evidence-based toxicology as part of the larger movement towards evidence-based practices. Toxicology is currently contributing to the field of cancer research, since some toxins can be used as drugs for killing tumor cells. One prime example of this is ribosome-inactivating proteins, tested in the treatment of leukemia. The word ''toxicology'' () is a neoclas ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
BASF
BASF Societas Europaea, SE () is a German multinational corporation, multinational chemical company and the List of largest chemical producers, largest chemical producer in the world. Its headquarters is located in Ludwigshafen, Germany. The BASF Group comprises subsidiary, subsidiaries and joint ventures in more than 80 countries and operates six integrated production sites and 390 other production sites in Europe, Asia, Australia, the Americas and Africa. BASF has customers in over 190 countries and supplies products to a wide variety of industries. Despite its size and global presence, BASF has received relatively little public attention since it abandoned the manufacture and sale of BASF-branded consumer electronics products in the 1990s. At the end of 2019, the company employed 117,628 people, with over 54,000 in Germany. , BASF posted sales of €59.3 billion and income from operations before special items of about €4.5 billion. Between 1990 and 2005, the co ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Regulatory Toxicology And Pharmacology
''Regulatory Toxicology and Pharmacology'' is a monthly peer-reviewed scientific journal which covers legal aspects of toxicological and pharmacological regulations. It is published by Elsevier on behalf of the International Society of Regulatory Toxicology & Pharmacology. The current co-editors-in-chief are Lesa L. Aylward and Martin van den Berg. Conflicts of interest In 2002, a group of 45 academics wrote a letter accusing the journal of a concealed pro-industry bias, a possible lack of full and independent peer review, and a failure to disclose conflicts of interest, citing a case in which Gio Batta Gori, then-current editor-in-chief, was paid $30,000 by the Tobacco Institute to write an article later published in the journal dismissing the health risks of secondhand smoke. The letter's coordinator later commented that the journal "reads like an industry trade publication, but it's masked as a peer-reviewed journal" and that it lacked any "credible peer-review process." In r ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
OECD Guidelines For The Testing Of Chemicals
OECD Guidelines for the Testing of Chemicals (OECD TG) are a set of internationally accepted specifications for the testing of chemicals decided on by the Organisation for Economic Co-operation and Development (OECD). They were first published in 1981. They are split into five sections: * Section 1: Physical Chemical Properties * Section 2: Effects on Biotic Systems * Section 3: Environmental Fate and Behaviour * Section 4: Health Effects * Section 5: Other Test Guidelines Guidelines are numbered with three digit numbers, the section number being the first number. Sometimes guidelines are suffixed with a letter. Guidelines are under constant review, with guidelines being periodically updated, new guidelines being adopted, and guidelines being withdrawn. Previous guidelines are maintained on the website for reference purposes. Animal welfare concerns are dealt with by ensuring that animal tests are only permitted where necessary. The guidelines are available in both English ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
EU Test Methods
The Test Methods Regulation is a Regulation (European Union) No. 440/2008 of May 30, 2008. It, and its subsequent amendments, define tests, testing of chemicals for the REACH Regulation. They are based on the OECD Guidelines for the Testing of Chemicals OECD Guidelines for the Testing of Chemicals (OECD TG) are a set of internationally accepted specifications for the testing of chemicals decided on by the Organisation for Economic Co-operation and Development (OECD). They were first published in .... External links * * https://web.archive.org/web/20110928164636/http://ihcp.jrc.ec.europa.eu/our_activities/alt-animal-testing/test_method_reg/ {{EU-stub Regulation of chemicals Toxicology European Union regulations ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Good Laboratory Practice
In the experimental (non-clinical) research arena, good laboratory practice or GLP is a quality system of management controls for research laboratories and organizations to ensure the uniformity, consistency, reliability, reproducibility, quality, and integrity of products in development for human or animal health (including pharmaceuticals) through non-clinical safety tests; from physio-chemical properties through acute to chronic toxicity tests. GLP was first introduced in New Zealand and Denmark in 1972, and later in the US in 1978 in response to the Industrial BioTest Labs scandal. It was followed a few years later by the Organization for Economic Co-operation and Development (OECD) Principles of GLP in 1992; the OECD has since helped promulgate GLP to many countries. GLP applies to non-clinical studies conducted for the assessment of the safety or efficacy of products in development (including pharmaceuticals) for people, animals, and the environment. GLP, a data and opera ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
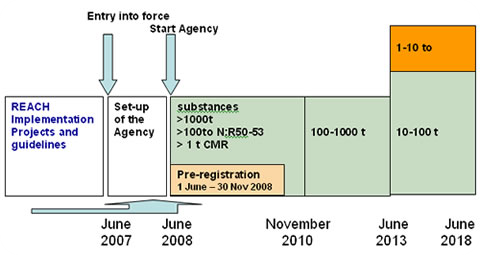
REACH Regulation
Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH) is a European Union regulation dating from 18 December 2006. REACH addresses the production and use of chemical substances, and their potential impacts on both human health and the environment. Its 849 pages took seven years to pass, and it has been described as the most complex legislation in the Union's history and the most important in 20 years. It is the strictest law to date regulating chemical substances and will affect industries throughout the world. REACH entered into force on 1 June 2007, with a phased implementation over the next decade. The regulation also established the European Chemicals Agency, which manages the technical, scientific and administrative aspects of REACH. Overview When REACH is fully in force, it will require all companies manufacturing or importing chemical substances into the European Union in quantities of one tonne or more per year to register these substances with a n ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
IUCLID
IUCLID (; International Uniform Chemical Information Database) is a software application to capture, store, maintain and exchange data on intrinsic and hazard properties of chemical substances. Distributed free of charge, the software is especially useful to chemical industry companies and to government authorities. It is the key tool for chemical industry to fulfill data submission obligations under REACH, the most important European Union legal document covering the production and use of chemical substances. The software is maintained by the European Chemicals Agency, ECHA. The latest version, version 6, was made available on 29 April 2016. History IUCLID versions 1 to 4 1993: First version of IUCLID for the European Existing Substances Regulation 793/93/EEC. 1999: IUCLID becomes the recommended tool for the OECD HPV Programme. 2000: IUCLID is the software prescribed in the EU Biocides legislation to notify existing active substances (Art. 4 of Commission Regulation (EC) N ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
European Chemicals Agency
The European Chemicals Agency (ECHA; ) is an agency of the European Union which manages the technical and administrative aspects of the implementation of the European Union regulation called Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH). ECHA is the driving force among regulatory authorities in implementing the EU's chemicals legislation. ECHA has to ascertain that companies comply with the legislation, advances the safe use of chemicals, provides information on chemicals and addresses chemicals of concern. It is located in Helsinki, Finland. ECHA is an independent and mature regulatory agency established by REACH. It is not a subsidiary entity of the European Commission. The agency, currently headed by Acting Executive Director Shay O’Malley, started working on 1 June 2007. Establishment The ECHA was created by European Union regulation dating from 18 December 2006 to manage the then-new legislation to regulate the manufacture and use of chemic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Randomization
Randomization is the process of making something random. Randomization is not haphazard; instead, a random process is a sequence of random variables describing a process whose outcomes do not follow a deterministic pattern, but follow an evolution described by probability distributions. For example, a random sample of individuals from a population refers to a sample where every individual has a known probability of being sampled. This would be contrasted with nonprobability sampling where arbitrary individuals are selected. In various contexts, randomization may involve: * generating a random permutation of a sequence (such as when shuffling cards); * selecting a random sample of a population (important in statistical sampling); * allocating experimental units via random assignment to a treatment or control condition; * generating random numbers (random number generation); or * transforming a data stream (such as when using a scrambler in telecommunications). Applications Randomi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Blinded Experiment
In a blind or blinded experiment, information which may influence the participants of the experiment is withheld until after the experiment is complete. Good blinding can reduce or eliminate experimental biases that arise from a participants' expectations, observer's effect on the participants, observer bias, confirmation bias, and other sources. A blind can be imposed on any participant of an experiment, including subjects, researchers, technicians, data analysts, and evaluators. In some cases, while blinding would be useful, it is impossible or unethical. For example, it is not possible to blind a patient to their treatment in a physical therapy intervention. A good clinical protocol ensures that blinding is as effective as possible within ethical and practical constraints. During the course of an experiment, a participant becomes unblinded if they deduce or otherwise obtain information that has been masked to them. For example, a patient who experiences a side effect may corr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
_(6009043040).jpg)