|
Kalkitoxin
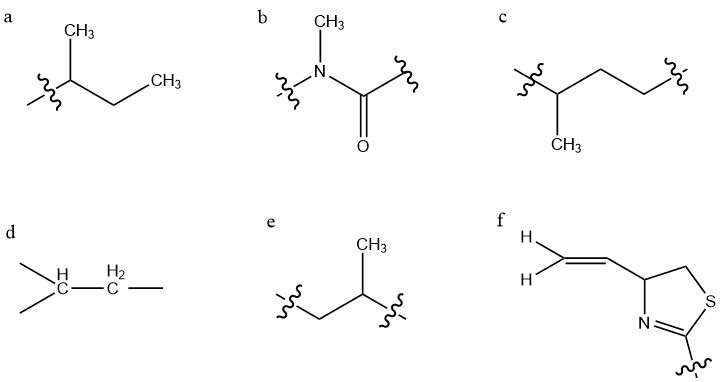
Kalkitoxin, a toxin derived from the cyanobacterium ''Lyngbya majuscula'', induces NMDA receptor mediated neuronal necrosis, blocks voltage-dependent sodium channels, and induces cellular hypoxia by inhibiting the electron transport chain (ETC) complex 1. Natural sources Kalkitoxin is an ichthyotoxin, derived from the cyanobacterium ''Lyngbya majuscula'' which covers sections of the coral reef. It typically forms mini-blooms and produces several metabolites, such as kalkitoxin, curacin-A and antillatoxin. Kalkitoxin has been found and purified near the coasts of Curaçao and Puerto Rico. Structure and reactivity Kalkitoxin is a lipopeptide toxin with a molecular weight of 366.604Da. Its chemical formula is C21H38N2OS. The structure contains two double bonds, a 2,4-disubstituted thiazoline ring system, and an additional carbonyl-group. These four groups each provide a degree of unsaturation, which causes kalkitoxin to have four degrees of unsaturation. The structure cont ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lyngbya Majuscula
''Lyngbya majuscula'' is a species of filamentous cyanobacteria in the genus ''Lyngbya''. It is named after the Dane Hans Christian Lyngbye. As a result of recent genetic analyses, several new genera were erected from the genus ''Lyngbya'': ''e.g.'', ''Moorea Moorea ( or ; Tahitian: ), also spelled Moorea, is a volcanic island in French Polynesia. It is one of the Windward Islands, a group that is part of the Society Islands, northwest of Tahiti. The name comes from the Tahitian word , meaning ...'', '' Limnoraphis'', '' Okeania'', '' Microseira'', and '' Dapis''. Almost 300 different secondary metabolites have been isolated from specimens identified as ''L. majuscula''. However, most of these studies lack a molecular identification of the samples. Several specimens identified as ''L. majuscula'' and collected in marine tropical regions are now classified as members of the genera ''Okeania'' and ''Moorea''. Antillatoxin and Kalkitoxin have been reported to be extr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Toxin
A toxin is a naturally occurring organic poison produced by metabolic activities of living cells or organisms. Toxins occur especially as a protein or conjugated protein. The term toxin was first used by organic chemist Ludwig Brieger (1849–1919) and is derived from the word toxic. Toxins can be small molecules, peptides, or proteins that are capable of causing disease on contact with or absorption by body tissues interacting with biological macromolecules such as enzymes or cellular receptors. Toxins vary greatly in their toxicity, ranging from usually minor (such as a bee sting) to potentially fatal even at extremely low doses (such as botulinum toxin). Toxins are largely secondary metabolites, which are organic compounds that are not directly involved in an organism's growth, development, or reproduction, instead often aiding it in matters of defense. Terminology Toxins are often distinguished from other chemical agents strictly based on their biological origin. Le ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Butyl Group
In organic chemistry, butyl is a four-carbon alkyl radical or substituent group with general chemical formula , derived from either of the two isomers (''n''-butane and isobutane) of butane. The isomer ''n''-butane can connect in two ways, giving rise to two "-butyl" groups: * If it connects at one of the two terminal carbon atoms, it is normal butyl or ''n''-butyl: (preferred IUPAC name: butyl) * If it connects at one of the non-terminal (internal) carbon atoms, it is secondary butyl or ''sec''-butyl: (preferred IUPAC name: butan-2-yl) The second isomer of butane, isobutane, can also connect in two ways, giving rise to two additional groups: * If it connects at one of the three terminal carbons, it is isobutyl: (preferred IUPAC name: 2-methylpropyl) * If it connects at the central carbon, it is tertiary butyl, ''tert''-butyl or ''t''-butyl: (preferred IUPAC name: ''tert''-butyl) Nomenclature According to IUPAC nomenclature, "isobutyl", "''sec''-butyl", and "''tert''-b ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mass Spectrometry
Mass spectrometry (MS) is an analytical technique that is used to measure the mass-to-charge ratio of ions. The results are presented as a ''mass spectrum'', a plot of intensity as a function of the mass-to-charge ratio. Mass spectrometry is used in many different fields and is applied to pure samples as well as complex mixtures. A mass spectrum is a type of plot of the ion signal as a function of the mass-to-charge ratio. These spectra are used to determine the elemental or isotopic signature of a sample, the masses of particles and of molecules, and to elucidate the chemical identity or structure of molecules and other chemical compounds. In a typical MS procedure, a sample, which may be solid, liquid, or gaseous, is ionized, for example by bombarding it with a beam of electrons. This may cause some of the sample's molecules to break up into positively charged fragments or simply become positively charged without fragmenting. These ions (fragments) are then separated accordin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electron Ionization
Electron ionization (EI, formerly known as electron impact ionization and electron bombardment ionization) is an ionization method in which energetic electrons interact with solid or gas phase atoms or molecules to produce ions. EI was one of the first ionization techniques developed for mass spectrometry. However, this method is still a popular ionization technique. This technique is considered a hard (high fragmentation) ionization method, since it uses highly energetic electrons to produce ions. This leads to extensive fragmentation, which can be helpful for structure determination of unknown compounds. EI is the most useful for organic compounds which have a molecular weight below 600. Also, several other thermally stable and volatile compounds in solid, liquid and gas states can be detected with the use of this technique when coupled with various separation methods. History Electron ionization was first described in 1918 by Canadian-American Physicist Arthur J. Dempste ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alkene
In organic chemistry, an alkene is a hydrocarbon containing a carbon–carbon double bond. Alkene is often used as synonym of olefin, that is, any hydrocarbon containing one or more double bonds.H. Stephen Stoker (2015): General, Organic, and Biological Chemistry'. 1232 pages. Two general types of monoalkenes are distinguished: terminal and internal. Also called α-olefins, terminal alkenes are more useful. However, the International Union of Pure and Applied Chemistry (IUPAC) recommends using the name "alkene" only for acyclic hydrocarbons with just one double bond; alkadiene, alkatriene, etc., or polyene for acyclic hydrocarbons with two or more double bonds; cycloalkene, cycloalkadiene, etc. for cyclic ones; and "olefin" for the general class – cyclic or acyclic, with one or more double bonds. Acyclic alkenes, with only one double bond and no other functional groups (also known as mono-enes) form a homologous series of hydrocarbons with the general formula with '' ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
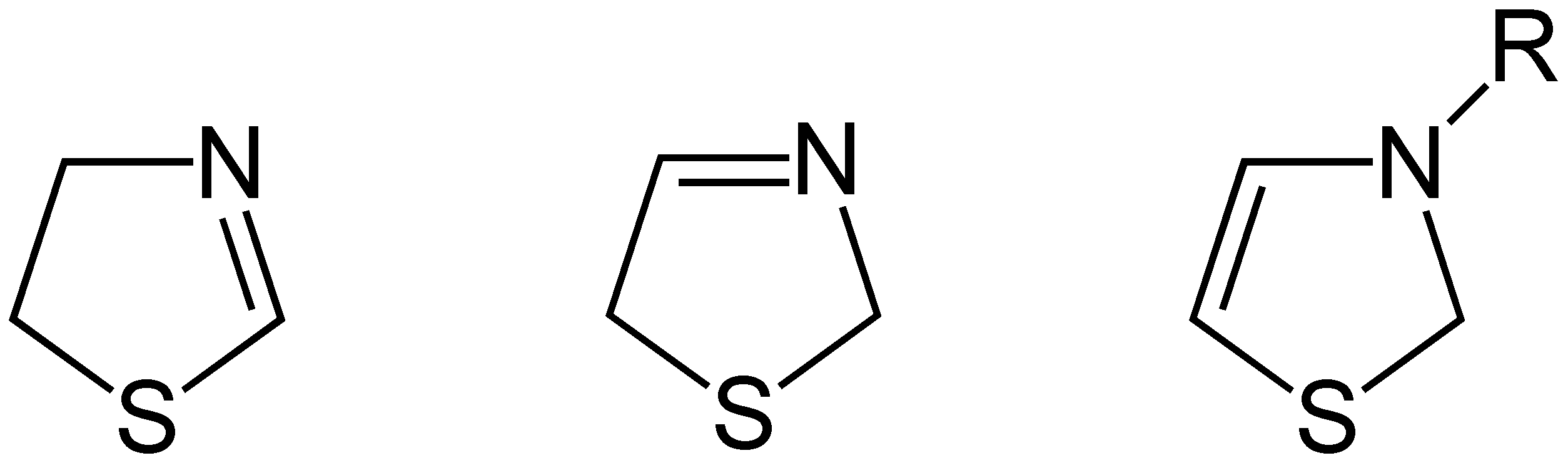
Thiazoline
Thiazolines (or dihydrothiazoles) are a group of isomeric 5-membered heterocyclic compounds containing both sulfur and nitrogen in the ring. Although unsubstituted thiazolines are rarely encountered themselves, their derivatives are more common and some are bioactive. For example, in a common post-translational modification, cysteine residues are converted into thiazolines. The name thiazoline originates from the Hantzsch–Widman nomenclature. Isomers Three structural isomers of thiazoline exist depending on the position of the double bond. These forms do not readily interconvert and hence are not tautomers. Of these 2-thiazoline is the most common. A fourth structure exists in which the N and S atoms are adjacent; this known as isothiazoline. Synthesis Thiazolines were first prepared by dialkylation of thioamides by Richard Willstatter in 1909. 2-Thiazolines are commonly prepared from 2-aminoethanethiols (e.g. cysteamine). They may also be synthesized via the Asinger r ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Shift
In nuclear magnetic resonance (NMR) spectroscopy, the chemical shift is the resonant frequency of an atomic nucleus relative to a standard in a magnetic field. Often the position and number of chemical shifts are diagnostic of the structure of a molecule. Chemical shifts are also used to describe signals in other forms of spectroscopy such as photoemission spectroscopy. Some atomic nuclei possess a magnetic moment ( nuclear spin), which gives rise to different energy levels and resonance frequencies in a magnetic field. The total magnetic field experienced by a nucleus includes local magnetic fields induced by currents of electrons in the molecular orbitals (note that electrons have a magnetic moment themselves). The electron distribution of the same type of nucleus (e.g. ) usually varies according to the local geometry (binding partners, bond lengths, angles between bonds, and so on), and with it the local magnetic field at each nucleus. This is reflected in the spin energy le ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electron Density
In quantum chemistry, electron density or electronic density is the measure of the probability of an electron being present at an infinitesimal element of space surrounding any given point. It is a scalar quantity depending upon three spatial variables and is typically denoted as either \rho(\textbf r) or n(\textbf r). The density is determined, through definition, by the normalised N-electron wavefunction which itself depends upon 4N variables (3N spatial and N spin coordinates). Conversely, the density determines the wave function modulo up to a phase factor, providing the formal foundation of density functional theory. According to quantum mechanics, due to the uncertainty principle on an atomic scale the exact location of an electron cannot be predicted, only the probability of its being at a given position; therefore electrons in atoms and molecules act as if they are "smeared out" in space. For one-electron systems, the electron density at any point is proportional to th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electronegativity
Electronegativity, symbolized as , is the tendency for an atom of a given chemical element to attract shared electrons (or electron density) when forming a chemical bond. An atom's electronegativity is affected by both its atomic number and the distance at which its valence electrons reside from the charged nucleus. The higher the associated electronegativity, the more an atom or a substituent group attracts electrons. Electronegativity serves as a simple way to quantitatively estimate the bond energy, and the sign and magnitude of a bond's chemical polarity, which characterizes a bond along the continuous scale from covalent to ionic bonding. The loosely defined term electropositivity is the opposite of electronegativity: it characterizes an element's tendency to donate valence electrons. On the most basic level, electronegativity is determined by factors like the nuclear charge (the more protons an atom has, the more "pull" it will have on electrons) and the number and location ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Imine
In organic chemistry, an imine ( or ) is a functional group or organic compound containing a carbon–nitrogen double bond (). The nitrogen atom can be attached to a hydrogen or an organic group (R). The carbon atom has two additional single bonds. Imines are common in synthetic and naturally occurring compounds and they participate in many reactions. Structure For ketimines and aldimines, respectively, the five core atoms (C2C=NX and C(H)C=NX, X = H or C) are coplanar. Planarity results from the sp2-hybridization of the mutually double-bonded carbon and the nitrogen atoms. The C=N distance is 1.29-1.31 Å for nonconjugated imines and 1.35 Å for conjugated imines. By contrast, C-N distances in amines and nitriles are 1.47 and 1.16 Å, respectively. Rotation about the C=N bond is slow. Using NMR spectroscopy, both E- and Z-isomers of aldimines have been detected. Owing to steric effects, the E isomer is favored. Nomenclature and classification The term "imine" was coine ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Proton
A proton is a stable subatomic particle, symbol , H+, or 1H+ with a positive electric charge of +1 ''e'' elementary charge. Its mass is slightly less than that of a neutron and 1,836 times the mass of an electron (the proton–electron mass ratio). Protons and neutrons, each with masses of approximately one atomic mass unit, are jointly referred to as "nucleons" (particles present in atomic nuclei). One or more protons are present in the nucleus of every atom. They provide the attractive electrostatic central force which binds the atomic electrons. The number of protons in the nucleus is the defining property of an element, and is referred to as the atomic number (represented by the symbol ''Z''). Since each element has a unique number of protons, each element has its own unique atomic number, which determines the number of atomic electrons and consequently the chemical characteristics of the element. The word ''proton'' is Greek for "first", and this name was given to the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |

.jpg)