|
Kipp's Apparatus
Kipp's apparatus, also called Kipp generator, is an apparatus designed for preparation of small volumes of gases. It was invented around 1844 by the Dutch pharmacist Petrus Jacobus Kipp and widely used in chemical laboratories and for demonstrations in schools into the second half of the 20th century. It later fell out of use, at least in laboratories, because most gases then became available in small gas cylinders. These industrial gases are much purer and drier than those initially obtained from a Kipp apparatus without further processing. Design and operation The apparatus is usually made of glass, or sometimes of polyethylene, and consists of three vertically stacked chambers, roughly resembling a snowman. The upper chamber extends downward as a tube that passes through the middle chamber into the lower chamber. There is no direct path between the middle and upper chambers, but the middle chamber is separated from the lower chamber by a retention plate, such as a conical pi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Kipp Apparat
The Knowledge is Power Program, commonly known as KIPP, is a network of free open-enrollment college-preparatory schools in low income communities throughout the United States. KIPP is America's largest network of charter schools. The head offices are in San Francisco, Chicago, New York City, and Washington, D.C. KIPP was founded in 1994 by Mike Feinberg and Dave Levin, two Teach For America corps members, influenced by Harriett Ball. KIPP was one of the charter school organizations to help produce the Relay Graduate School of Education for teacher training. History KIPP began in 1994 after co-founders Dave Levin and Mike Feinberg completed their two-year commitment to Teach For America. A year later, they launched a program for fifth graders in a public school in inner-city Houston, Texas. Feinberg developed KIPP Academy Houston into a charter school, while Levin went on to establish KIPP Academy New York in the South Bronx. In February 2018, Feinberg was removed from ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Iron(II) Sulfide
Iron(II) sulfide or ferrous sulfide (Br.E. sulphide) is one of a family chemical compounds and minerals with the approximate formula . Iron sulfides are often iron-deficient non-stoichiometric. All are black, water-insoluble solids. Preparation and structure FeS can be obtained by the heating of iron and sulfur: :Fe + S → FeS FeS adopts the nickel arsenide structure, featuring octahedral Fe centers and trigonal prismatic sulfide sites. Reactions Iron sulfide reacts with hydrochloric acid, releasing hydrogen sulfide: :FeS + 2 HCl → FeCl2 + H2S :FeS + H2SO4 → FeSO4 + H2S In moist air, iron sulfides oxidize to hydrated ferrous sulfate. Biology and biogeochemistry Iron sulfides occur widely in nature in the form of iron–sulfur proteins. As organic matter decays under low-oxygen (or hypoxic) conditions such as in swamps or dead zones of lakes and oceans, sulfate-reducing bacteria reduce various sulfates present in the water, producing hydrogen sulfide. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
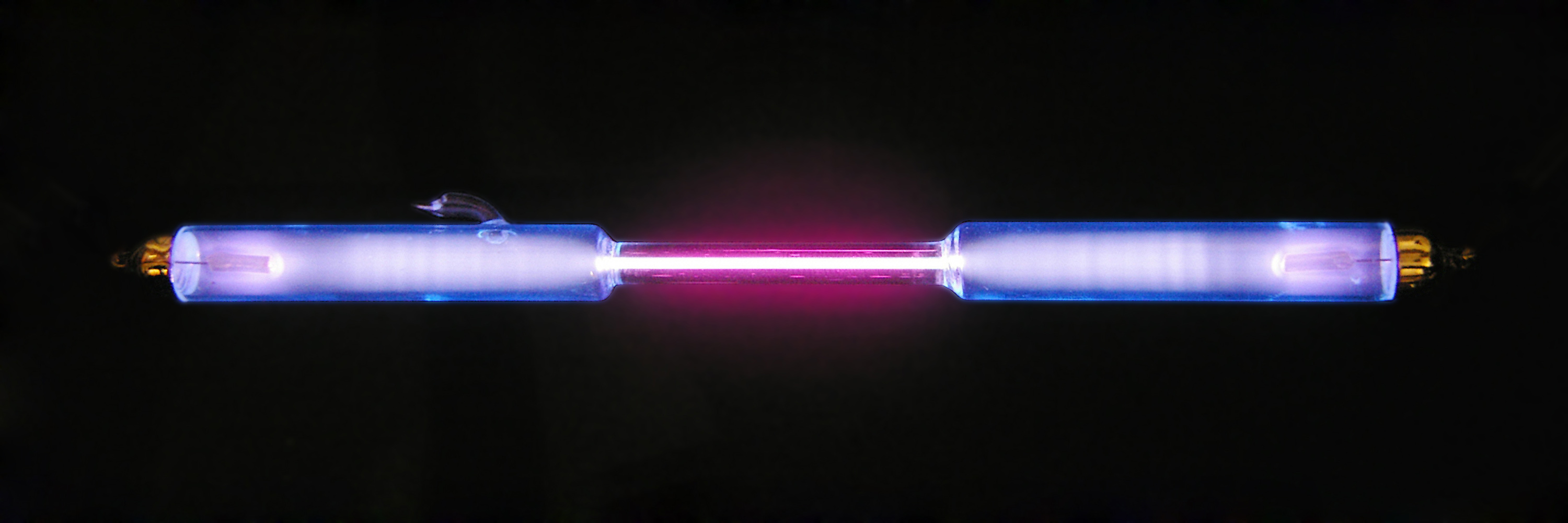
Oxygen
Oxygen is the chemical element with the symbol O and atomic number 8. It is a member of the chalcogen group in the periodic table, a highly reactive nonmetal, and an oxidizing agent that readily forms oxides with most elements as well as with other compounds. Oxygen is Earth's most abundant element, and after hydrogen and helium, it is the third-most abundant element in the universe. At standard temperature and pressure, two atoms of the element bind to form dioxygen, a colorless and odorless diatomic gas with the formula . Diatomic oxygen gas currently constitutes 20.95% of the Earth's atmosphere, though this has changed considerably over long periods of time. Oxygen makes up almost half of the Earth's crust in the form of oxides.Atkins, P.; Jones, L.; Laverman, L. (2016).''Chemical Principles'', 7th edition. Freeman. Many major classes of organic molecules in living organisms contain oxygen atoms, such as proteins, nucleic acids, carbohydrates, and fats, as ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Barium Ferrate
Barium ferrate is the chemical compound of formula BaFeO4. This is a rare compound containing iron in the +6 oxidation state. The ferrate(VI) ion has two unpaired electrons, making it paramagnetic. It is isostructural with BaSO4, and contains the tetrahedral FeO4/nowiki>.html" ;"title="/nowiki>FeO4/nowiki>">/nowiki>FeO4/nowiki>2− anion. Structure The ferrate(VI) anion is paramagnetic due to its two unpaired electrons and it has a tetrahedral molecular geometry. X-ray diffraction has been used to determine the orthorhombic unit cell structure (lattice vectors a ≠ b ≠ c, interaxial angles α=β=γ=90°) of nanocrystalline BaFeO4. It crystallized in the Pnma space group (point group: D2h) with lattice parameters ''a'' = 0.8880 nm, ''b'' = 0.5512 nm and ''c'' = 0.7214 nm. The accuracy of the X-Ray diffraction data has been verified by the lattice fringe intervals from High-Resolution Transmission Electron Microscopy (HRTEM) and cell parameters calculated from ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Manganese Dioxide
Manganese dioxide is the inorganic compound with the formula . This blackish or brown solid occurs naturally as the mineral pyrolusite, which is the main ore of manganese and a component of manganese nodules. The principal use for is for dry-cell batteries, such as the alkaline battery and the zinc–carbon battery.. is also used as a pigment and as a precursor to other manganese compounds, such as . It is used as a reagent in organic synthesis, for example, for the oxidation of allylic alcohols. is α polymorph that can incorporate a variety of atoms (as well as water molecules) in the "tunnels" or "channels" between the manganese oxide octahedra. There is considerable interest in as a possible cathode for lithium-ion batteries. Structure Several polymorphs of are claimed, as well as a hydrated form. Like many other dioxides, crystallizes in the rutile crystal structure (this polymorph is called pyrolusite or ), with three-coordinate oxide and octahedral metal centres. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Calcium Hypochlorite
Calcium hypochlorite is an inorganic compound with formula Ca(OCl)2. It is the main active ingredient of commercial products called bleaching powder, chlorine powder, or chlorinated lime, used for water treatment and as a bleaching agent. This compound is relatively stable and has greater available chlorine than sodium hypochlorite. It is a white solid, although commercial samples appear yellow. It strongly smells of chlorine, owing to its slow decomposition in moist air. History Charles Tennant and Charles Macintosh developed an industrial process for the manufacture of Chloride of Lime in the late 18th Century. It was patented in 1799 and used heavily during World War I for disinfecting the trenches and wounds. Uses Sanitation Calcium hypochlorite is commonly used to sanitize public swimming pools and disinfect drinking water. Generally the commercial substances are sold with a purity of 65% to 73% with other chemicals present, such as calcium chloride and calcium carb ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Potassium Permanganate
Potassium permanganate is an inorganic compound with the chemical formula KMnO4. It is a purplish-black crystalline salt, that dissolves in water as K+ and , an intensely pink to purple solution. Potassium permanganate is widely used in the chemical industry and laboratories as a strong oxidizing agent, and also as a medication for dermatitis, for cleaning wounds, and general disinfection. It is on the World Health Organization's List of Essential Medicines. In 2000, worldwide production was estimated at 30,000 tonnes. Properties Potassium permanganate is the potassium salt of the tetrahedral transition metal oxo complex permanganate, in which four O2- ligands are bound to a manganese(VII) center. Structure KMnO4 forms orthorhombic crystals with constants: ''a'' = 910.5 pm, ''b'' = 572.0 pm, ''c'' = 742.5 pm. The overall motif is similar to that for barium sulfate, with which it forms solid solutions. In the solid (as in solution), each MnO4− centre is t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chlorine
Chlorine is a chemical element with the Symbol (chemistry), symbol Cl and atomic number 17. The second-lightest of the halogens, it appears between fluorine and bromine in the periodic table and its properties are mostly intermediate between them. Chlorine is a yellow-green gas at room temperature. It is an extremely reactive element and a strong oxidising agent: among the elements, it has the highest electron affinity and the third-highest electronegativity on the revised Electronegativity#Pauling electronegativity, Pauling scale, behind only oxygen and fluorine. Chlorine played an important role in the experiments conducted by medieval Alchemy, alchemists, which commonly involved the heating of chloride Salt (chemistry), salts like ammonium chloride (sal ammoniac) and sodium chloride (common salt), producing various chemical substances containing chlorine such as hydrogen chloride, mercury(II) chloride (corrosive sublimate), and hydrochloric acid (in the form of ). However ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Deuterium
Deuterium (or hydrogen-2, symbol or deuterium, also known as heavy hydrogen) is one of two Stable isotope ratio, stable isotopes of hydrogen (the other being Hydrogen atom, protium, or hydrogen-1). The atomic nucleus, nucleus of a deuterium atom, called a deuteron, contains one proton and one neutron, whereas the far more common protium has no neutrons in the nucleus. Deuterium has a natural abundance in Earth's oceans of about one atom of deuterium among all atoms of hydrogen (see heavy water). Thus deuterium accounts for approximately 0.0156% by number (0.0312% by mass) of all the naturally occurring hydrogen in the oceans, while protium accounts for more than 99.98%. The abundance of deuterium changes slightly from one kind of natural water to another (see Vienna Standard Mean Ocean Water). (Tritium is yet another hydrogen isotope, with two neutrons, that is far more rare and is radioactive.) The name ''deuterium'' is derived from the Greek , meaning "second", to denot ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aluminium Carbide
Aluminum carbide, chemical formula Al4 C3, is a carbide of aluminum. It has the appearance of pale yellow to brown crystals. It is stable up to 1400 °C. It decomposes in water with the production of methane. Structure Aluminum carbide has an unusual crystal structure that consists of alternating layers of Al2C and Al2C2. Each aluminum atom is coordinated to 4 carbon atoms to give a tetrahedral arrangement. Carbon atoms exist in 2 different binding environments; one is a deformed octahedron of 6 Al atoms at a distance of 217 pm. The other is a distorted trigonal bipyramidal structure of 4 Al atoms at 190–194 pm and a fifth Al atom at 221 pm. Other carbides (IUPAC nomenclature: methides) also exhibit complex structures. Reactions Aluminum carbide hydrolyses with evolution of methane. The reaction proceeds at room temperature but is rapidly accelerated by heating. : Al4C3 + 12 H2O → 4 Al(OH)3 + 3 CH4 Similar reactions occur with other protic reagents: : Al4C3 + 12 HCl � ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Methane
Methane ( , ) is a chemical compound with the chemical formula (one carbon atom bonded to four hydrogen atoms). It is a group-14 hydride, the simplest alkane, and the main constituent of natural gas. The relative abundance of methane on Earth makes it an economically attractive fuel, although capturing and storing it poses technical challenges due to its gaseous state under normal conditions for temperature and pressure. Naturally occurring methane is found both below ground and under the seafloor and is formed by both geological and biological processes. The largest reservoir of methane is under the seafloor in the form of methane clathrates. When methane reaches the surface and the atmosphere, it is known as atmospheric methane. The Earth's atmospheric methane concentration has increased by about 150% since 1750, and it accounts for 20% of the total radiative forcing from all of the long-lived and globally mixed greenhouse gases. It has also been detected on other plane ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |