|
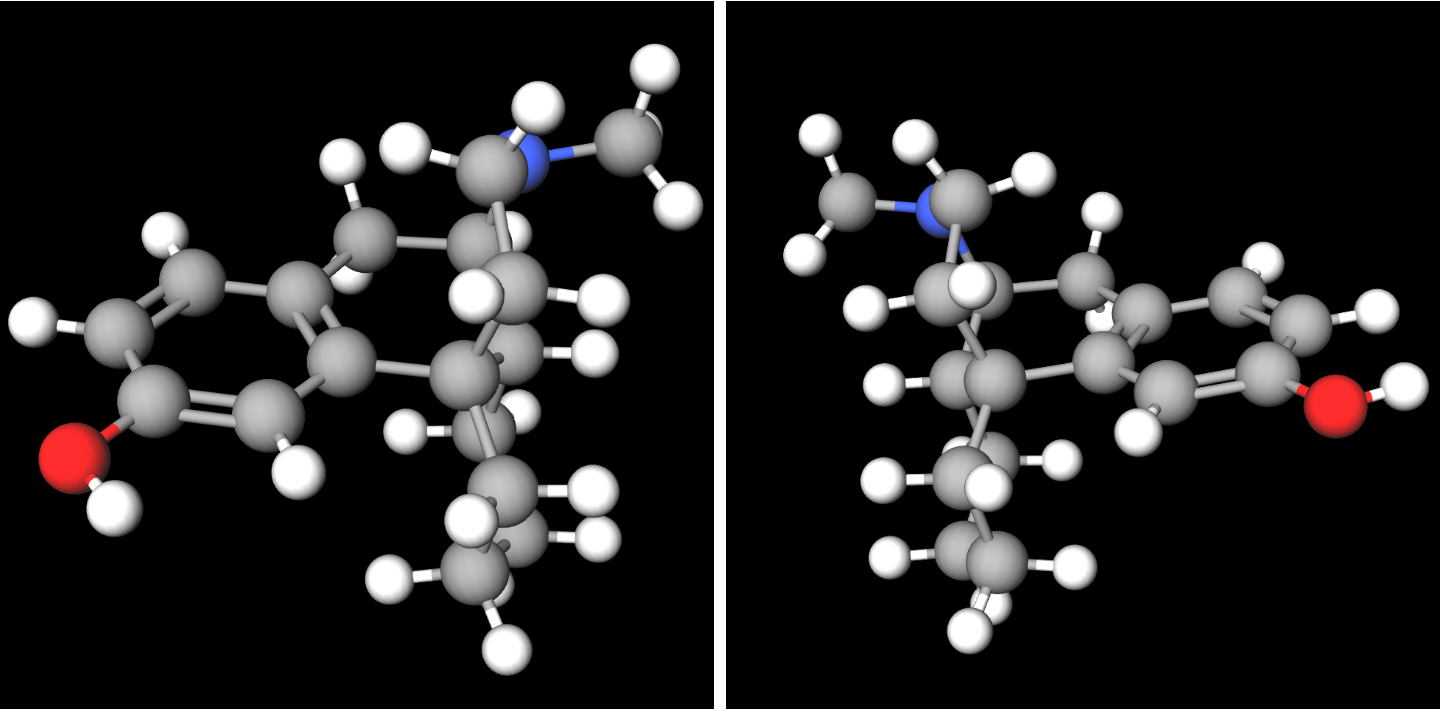
Ketorfanol
Ketorfanol (International Nonproprietary Name, INN, United States Adopted Name, USAN) (developmental code name SBW-22), or ketorphanol, is an opioid analgesic of the morphinan family that was found to possess "potent antinociception, antiwrithing activity" in animal research, animal assays but was never marketed. It is a 17-cycloalkylmethyl derivative of morphinan and as such, is closely related structurally to butorphanol, cyclorphan, oxilorphan, proxorphan, and xorphanol, which act preferentially as κ-opioid receptor agonists and to a lesser extent as μ-opioid receptor partial agonists/receptor antagonist, antagonists. See also * Butorphanol * Levallorphan * Levomethorphan * Levorphanol * Nalbuphine * Xorphanol References Phenols Analgesics Ketones Morphinans Opioids {{analgesic-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Morphinans
Morphinan is the prototype chemical structure of a large chemical class of psychoactive drugs, consisting of opiate analgesics, cough suppressants, and dissociative hallucinogens, among others. Structure Morphinan has a phenanthrene core structure with the ''A'' ring remaining aromatic and the ''B'' and ''C'' rings being saturated, and an additional nitrogen-containing, six-membered, saturated ring, the ''D'' ring, being attached to carbons 9 and 13 of the core, and with the nitrogen being at position 17 of the composite. Of the major naturally occurring opiates of the morphinan type—morphine, codeine and thebaine—thebaine has no therapeutic properties (it causes seizures in mammals), but it provides a low-cost feedstock for the industrial production of at least four semi-synthetic opiate agonists, including hydrocodone, hydromorphone, oxycodone and oxymorphone, and the opioid antagonist naloxone. Structure-activity relationship The physiological behavior of morphinans ( ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Morphinan
Morphinan is the prototype chemical structure of a large chemical class of psychoactive drugs, consisting of opiate analgesics, cough suppressants, and dissociative hallucinogens, among others. Structure Morphinan has a phenanthrene core structure with the ''A'' ring remaining aromatic and the ''B'' and ''C'' rings being saturated, and an additional nitrogen-containing, six-membered, saturated ring, the ''D'' ring, being attached to carbons 9 and 13 of the core, and with the nitrogen being at position 17 of the composite. Of the major naturally occurring opiates of the morphinan type—morphine, codeine and thebaine—thebaine has no therapeutic properties (it causes seizures in mammals), but it provides a low-cost feedstock for the industrial production of at least four semi-synthetic opiate agonists, including hydrocodone, hydromorphone, oxycodone and oxymorphone, and the opioid antagonist naloxone. Structure-activity relationship The physiological behavior of morphinans ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Xorphanol
Xorphanol (INN) (developmental code name TR-5379 or TR-5379M), also known as xorphanol mesylate (USAN), is an opioid analgesic of the morphinan family that was never marketed. Xorphanol is a mixed agonist–antagonist of opioid receptors, acting preferentially as a high- efficacy partial agonist/near-full agonist of the κ-opioid receptor (Ki = 0.4 nM; EC50 = 3.3 nM; = 49%; = 0.84) and to a lesser extent as a partial agonist of the μ-opioid receptor (Ki = 0.25 nM; IC50 = 3.4 nM; = 29%) with lower relative intrinsic activity and marked antagonistic potential (including the ability to antagonize morphine-induced effects and induce opioid withdrawal in opioid-dependent individuals). The drug has also been found to act as an agonist of the δ-opioid receptor (Ki = 1.0 nM; IC50 = 8 nM; = 76%). Xorphanol produces potent analgesia, and was originally claimed to possess a minimal potential for dependence or abuse. Moreover, side effects in anima ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oxilorphan
Oxilorphan (INN, USAN) (developmental code name L-BC-2605) is an opioid antagonist of the morphinan family that was never marketed. It acts as a μ-opioid receptor (MOR) antagonist but a κ-opioid receptor (KOR) partial agonist, and has similar effects to naloxone and around the same potency as an MOR antagonist. Oxilorphan has some weak partial agonist actions at the MOR (with miosis, nausea, dizziness, and some euphoria observed) and can produce hallucinogenic/dissociative effects at sufficient doses, indicative of KOR activation. It was trialed for the treatment of opioid addiction, but was not developed commercially. The KOR agonist effects of oxilorphan are associated with dysphoria, which combined with its hallucinogenic effects, serve to limit its clinical usefulness; indeed, many patients who experienced these side effects refused to take additional doses in clinical trials. See also * Butorphanol * Cyclorphan * Ketorfanol * Levallorphan * Levomethorphan * Levorphanol * Na ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Proxorphan
Proxorphan ( INN), also known as proxorphan tartate ( USAN) (developmental code name BL-5572M), is an opioid analgesic and antitussive drug of the morphinan family that was never marketed. It acts preferentially as a κ-opioid receptor partial agonist and to a lesser extent as a μ-opioid receptor partial agonist. Synthesis Starting material for this preparation is ketoester 1, available by one of the classical benzomorphan syntheses. Condensation with the ylide from Triethyl phosphonoacetate ( HWE reaction) affords diester 2. Catalytic hydrogenation proceeds from the less hindered face to afford the corresponding saturated diester (3). The esters are then reduced by means of LiAlH4 to give the glycol (4); this undergoes internal ether formation on treatment with acid to form the pyran ring of 5. Von Braun reaction with BrCN (or ethyl chloroformate) followed by saponification of the intermediate leads to the 2° amine (6). This is converted to the cyclopropylmethyl deriva ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Xorphanol
Xorphanol (INN) (developmental code name TR-5379 or TR-5379M), also known as xorphanol mesylate (USAN), is an opioid analgesic of the morphinan family that was never marketed. Xorphanol is a mixed agonist–antagonist of opioid receptors, acting preferentially as a high- efficacy partial agonist/near-full agonist of the κ-opioid receptor (Ki = 0.4 nM; EC50 = 3.3 nM; = 49%; = 0.84) and to a lesser extent as a partial agonist of the μ-opioid receptor (Ki = 0.25 nM; IC50 = 3.4 nM; = 29%) with lower relative intrinsic activity and marked antagonistic potential (including the ability to antagonize morphine-induced effects and induce opioid withdrawal in opioid-dependent individuals). The drug has also been found to act as an agonist of the δ-opioid receptor (Ki = 1.0 nM; IC50 = 8 nM; = 76%). Xorphanol produces potent analgesia, and was originally claimed to possess a minimal potential for dependence or abuse. Moreover, side effects in anima ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Receptor Antagonist
A receptor antagonist is a type of receptor ligand or drug that blocks or dampens a biological response by binding to and blocking a receptor rather than activating it like an agonist. Antagonist drugs interfere in the natural operation of receptor proteins.Pharmacology Guide: In vitro pharmacology: concentration-response curves " '' GlaxoWellcome.'' Retrieved on December 6, 2007. They are sometimes called blockers; examples include alpha blockers, |
Ketones
In organic chemistry, a ketone is a functional group with the structure R–C(=O)–R', where R and R' can be a variety of carbon-containing substituents. Ketones contain a carbonyl group –C(=O)– (which contains a carbon-oxygen double bond C=O). The simplest ketone is acetone (where R and R' is methyl), with the formula . Many ketones are of great importance in biology and in industry. Examples include many sugars (ketoses), many steroids (e.g., testosterone), and the solvent acetone. Nomenclature and etymology The word ''ketone'' is derived from ''Aketon'', an old German word for ''acetone''. According to the rules of IUPAC nomenclature, ketone names are derived by changing the suffix ''-ane'' of the parent alkane to ''-anone''. Typically, the position of the carbonyl group is denoted by a number, but traditional nonsystematic names are still generally used for the most important ketones, for example acetone and benzophenone. These nonsystematic names are considered ret ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Analgesics
An analgesic drug, also called simply an analgesic (American English), analgaesic (British English), pain reliever, or painkiller, is any member of the group of drugs used to achieve relief from pain (that is, analgesia or pain management). It is typically used to induce cooperation with a medical procedure. Analgesics are conceptually distinct from anesthetics, which temporarily reduce, and in some instances eliminate, sensation, although analgesia and anesthesia are neurophysiologically overlapping and thus various drugs have both analgesic and anesthetic effects. Analgesic choice is also determined by the type of pain: For neuropathic pain, traditional analgesics are less effective, and there is often benefit from classes of drugs that are not normally considered analgesics, such as tricyclic antidepressants and anticonvulsants. Various analgesics, such as many NSAIDs, are available over the counter in most countries, whereas various others are prescription drugs owing to t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phenols
In organic chemistry, phenols, sometimes called phenolics, are a class of chemical compounds consisting of one or more hydroxyl groups (— O H) bonded directly to an aromatic hydrocarbon group. The simplest is phenol, . Phenolic compounds are classified as simple phenols or polyphenols based on the number of phenol units in the molecule. Phenols are both synthesized industrially and produced by plants and microorganisms. Properties Acidity Phenols are more acidic than typical alcohols. The acidity of the hydroxyl group in phenols is commonly intermediate between that of aliphatic alcohols and carboxylic acids (their pKa is usually between 10 and 12). Deprotonation of a phenol forms a corresponding negative phenolate ion or phenoxide ion, and the corresponding salts are called phenolates or phenoxides (aryloxides according to the IUPAC Gold Book). Condensation with aldehydes and ketones Phenols are susceptible to Electrophilic aromatic substitutions. Condensation with formald ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nalbuphine
Nalbuphine, sold under the brand names Nubain among others, is an opioid analgesic which is used in the treatment of pain. It is given by injection into a vein, muscle, or fat. Side effects of nalbuphine include sedation, sweatiness, clamminess, nausea, vomiting, dizziness, vertigo, dry mouth, and headache. Unlike other opioids, it has little to no capacity for euphoria or respiratory depression. It also has little to no incidence of dysphoria, dissociation, hallucinations, and related side effects at typical therapeutic doses. Nalbuphine is a mixed agonist/antagonist opioid modulator. Specifically, it acts as a moderate-efficacy partial agonist or antagonist of the μ-opioid receptor (MOR) and as a high-efficacy partial agonist of the κ-opioid receptor (KOR), whereas it has relatively low affinity for the δ-opioid receptor (DOR) and sigma receptors. Nalbuphine was patented in 1963US Patent 3393197 - Nusubstituted-14-hydroxydihydronormorphines and was introduced for medi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Levorphanol
Levorphanol (brand name Levo-Dromoran) is an opioid medication used to treat moderate to severe pain. It is the levorotatory enantiomer of the compound racemorphan. Its dextrorotatory counterpart is dextrorphan. It was first described in Germany in 1946. The drug has been in medical use in the United States since 1953. Pharmacology Levorphanol acts predominantly as an agonist of the μ-opioid receptor (MOR), but is also an agonist of the δ-opioid receptor (DOR), κ-opioid receptor (KOR), and the nociceptin receptor (NOP), as well as an NMDA receptor antagonist and a serotonin-norepinephrine reuptake inhibitor (SNRI). Levorphanol, similarly to certain other opioids, also acts as a glycine receptor antagonist and GABA receptor antagonist at very high concentrations. Levorphanol is 6 to 8 times as potent as morphine at the MOR. Relative to morphine, levorphanol lacks complete cross-tolerance and possesses greater intrinsic activity at the MOR. The duration of action is general ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |