|
Kapron Fishnet
Nylon 6 or polycaprolactam is a polymer, in particular semicrystalline polyamide. Unlike most other nylons, nylon 6 is not a condensation polymer, but instead is formed by ring-opening polymerization; this makes it a special case in the comparison between condensation and addition polymers. Its competition with nylon 6,6 and the example it set have also shaped the economics of the synthetic fibre industry. It is sold under numerous trade names including Perlon (Germany), Dederon (former East Germany), Nylatron, Capron, Ultramid, Akulon, Kapron (former Soviet Union and satellite states), Rugopa (Turkey) and Durethan. History Polycaprolactam was developed by Paul Schlack at IG Farben in late 1930s (first synthesized in 1938) to reproduce the properties of Nylon 66 without violating the patent on its production. (Around the same time, Kohei Hoshino at Toray also succeeded in synthesizing nylon 6.) It was marketed as Perlon, and industrial production with a capacity of 3,500 tons pe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nitrogen
Nitrogen is the chemical element with the symbol N and atomic number 7. Nitrogen is a nonmetal and the lightest member of group 15 of the periodic table, often called the pnictogens. It is a common element in the universe, estimated at seventh in total abundance in the Milky Way and the Solar System. At standard temperature and pressure, two atoms of the element bond to form N2, a colorless and odorless diatomic gas. N2 forms about 78% of Earth's atmosphere, making it the most abundant uncombined element. Nitrogen occurs in all organisms, primarily in amino acids (and thus proteins), in the nucleic acids ( DNA and RNA) and in the energy transfer molecule adenosine triphosphate. The human body contains about 3% nitrogen by mass, the fourth most abundant element in the body after oxygen, carbon, and hydrogen. The nitrogen cycle describes the movement of the element from the air, into the biosphere and organic compounds, then back into the atmosphere. Many indus ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
White Rot
A wood-decay or xylophagous fungus is any species of fungus that digests moist wood, causing it to rot. Some species of wood-decay fungi attack dead wood, such as brown rot, and some, such as ''Armillaria'' (honey fungus), are parasitic and colonize living trees. Excessive moisture above the fibre saturation point in wood is required for fungal colonization and proliferation. In nature, this process causes the breakdown of complex molecules and leads to the return of nutrients to the soil. Wood-decay fungi consume wood in various ways; for example, some attack the carbohydrates in wood and some others decay lignin. The rate of decay of wooden materials in various climates can be estimated by empirical models.Viitanen, T. et al. (2010). Towards modelling of decay risk of wooden materials. European Journal of Wood and Wood Products 68:303-313. Wood-decay fungi can be classified according to the type of decay that they cause. The best-known types are brown rot, soft rot, and whit ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oligomer
In chemistry and biochemistry, an oligomer () is a molecule that consists of a few repeating units which could be derived, actually or conceptually, from smaller molecules, monomers.Quote: ''Oligomer molecule: A molecule of intermediate relative molecular mass, the structure of which essentially comprises a small plurality of units derived, actually or conceptually, from molecules of lower relative molecular mass.'' The name is composed of Greek elements '' oligo-'', "a few" and '' -mer'', "parts". An adjective form is ''oligomeric''. The oligomer concept is contrasted to that of a polymer, which is usually understood to have a large number of units, possibly thousands or millions. However, there is no sharp distinction between these two concepts. One proposed criterion is whether the molecule's properties vary significantly with the removal of one or a few of the units. An oligomer with a specific number of units is referred to by the Greek prefix denoting that number, wi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
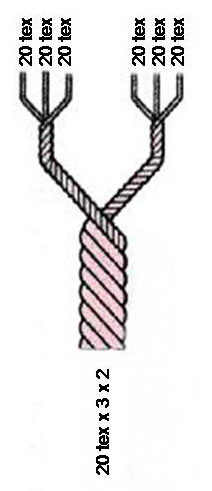
Units Of Textile Measurement
Textile fibers, threads, yarns and fabrics are measured in a multiplicity of units. * A fiber, a single filament of natural material, such as cotton, linen or wool, or artificial material such as nylon, polyester, metal or mineral fiber, or man-made cellulosic fibre like viscose, Modal, Lyocell or other rayon fiber is measured in terms of linear mass density, the weight of a given length of fiber. Various units are used to refer to the measurement of a fiber, such as: the denier and tex (linear mass density of fibers), super S (fineness of wool fiber), worsted count, woolen count, linen count (wet spun) (or Number English (Ne)), cotton count (or Number English (Ne)), Number metric (Nm) and yield (the reciprocal of denier and tex). * A yarn, a spun agglomeration of fibers used for knitting, weaving or sewing, is measured in terms of cotton count and yarn density. * Thread, usually consisting of multiple yarns plied together producing a long, thin strand used in sewing or weavi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gram-force
The kilogram-force (kgf or kgF), or kilopond (kp, from la, pondus, lit=weight), is a non-standard gravitational metric unit of force. It does not comply with the International System of Units (SI) and is deprecated for most uses. The kilogram-force is equal to the magnitude of the force exerted on one kilogram of mass in a gravitational field (standard gravity, a conventional value approximating the average magnitude of gravity on Earth). That is, it is the weight of a kilogram under standard gravity. Therefore, one kilogram-force is by definition equal to .NISTbr>''Guide for the Use of the International System of Units (SI)''Special Publication 811, (1995) page 51 Similarly, a gram-force is , and a milligram-force is . Kilogram-force is a non-standard unit and is classified in the International System of Units (SI) as a unit that is not accepted for use with SI. History The gram-force and kilogram-force were never well-defined units until the CGPM adopted a ''standard acceler ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tenacity (textile Strength)
The specific strength is a material's (or muscle's) Strength of materials, strength (force per unit area at failure) divided by its density. It is also known as the strength-to-weight ratio or strength/weight ratio or strength-to-mass ratio. In fiber or textile applications, Tenacity (textile strength), tenacity is the usual measure of specific strength. The SI unit for specific strength is Pascal (unit), Pa⋅metre, m3/kilogram, kg, or Newton (unit), N⋅m/kg, which is Dimensional analysis, dimensionally equivalent to m2/s2, though the latter form is rarely used. Specific strength has the same units as specific energy, and is related to the maximum specific energy of rotation that an object can have without flying apart due to centrifugal force. Another way to describe specific strength is breaking length, also known as self support length: the maximum length of a vertical column of the material (assuming a fixed cross-section) that could suspend its own weight when supported onl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nylon 6 And Nylon 6-6
Nylon is a generic designation for a family of synthetic polymers composed of polyamides ( repeating units linked by amide links).The polyamides may be aliphatic or semi-aromatic. Nylon is a silk-like thermoplastic, generally made from petroleum, that can be melt-processed into fibers, films, or shapes. Nylon polymers can be mixed with a wide variety of additives to achieve many property variations. Nylon polymers have found significant commercial applications in fabric and fibers (apparel, flooring and rubber reinforcement), in shapes (molded parts for cars, electrical equipment, etc.), and in films (mostly for food packaging). History DuPont and the invention of nylon Researchers at DuPont began developing cellulose based fibers, culminating in the synthetic fiber rayon. DuPont's experience with rayon was an important precursor to its development and marketing of nylon. DuPont's invention of nylon spanned an eleven-year period, ranging from the initial research progr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Amide
In organic chemistry, an amide, also known as an organic amide or a carboxamide, is a compound with the general formula , where R, R', and R″ represent organic groups or hydrogen atoms. The amide group is called a peptide bond when it is part of the main chain of a protein, and an isopeptide bond when it occurs in a side chain, such as in the amino acids asparagine and glutamine. It can be viewed as a derivative of a carboxylic acid () with the hydroxyl group () replaced by an amine group (); or, equivalently, an acyl (alkanoyl) group () joined to an amine group. Common examples of amides are acetamide (), benzamide (), and dimethylformamide (). Amides are qualified as primary, secondary, and tertiary according to whether the amine subgroup has the form , , or , where R and R' are groups other than hydrogen. The core of amides is called the amide group (specifically, carboxamide group). Amides are pervasive in nature and technology. Proteins and important plastics l ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Monomer
In chemistry, a monomer ( ; ''mono-'', "one" + '' -mer'', "part") is a molecule that can react together with other monomer molecules to form a larger polymer chain or three-dimensional network in a process called polymerization. Classification Monomers can be classified in many ways. They can be subdivided into two broad classes, depending on the kind of the polymer that they form. Monomers that participate in condensation polymerization have a different stoichiometry than monomers that participate in addition polymerization: : Other classifications include: *natural vs synthetic monomers, e.g. glycine vs caprolactam, respectively *polar vs nonpolar monomers, e.g. vinyl acetate vs ethylene, respectively *cyclic vs linear, e.g. ethylene oxide vs ethylene glycol, respectively The polymerization of one kind of monomer gives a homopolymer. Many polymers are copolymers, meaning that they are derived from two different monomers. In the case of condensation polymerizations, the r ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Molecule
A molecule is a group of two or more atoms held together by attractive forces known as chemical bonds; depending on context, the term may or may not include ions which satisfy this criterion. In quantum physics, organic chemistry, and biochemistry, the distinction from ions is dropped and ''molecule'' is often used when referring to polyatomic ions. A molecule may be homonuclear, that is, it consists of atoms of one chemical element, e.g. two atoms in the oxygen molecule (O2); or it may be heteronuclear, a chemical compound composed of more than one element, e.g. water (two hydrogen atoms and one oxygen atom; H2O). In the kinetic theory of gases, the term ''molecule'' is often used for any gaseous particle regardless of its composition. This relaxes the requirement that a molecule contains two or more atoms, since the noble gases are individual atoms. Atoms and complexes connected by non-covalent interactions, such as hydrogen bonds or ionic bonds, are typically not consid ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Caprolactam Polymerization
Caprolactam (CPL) is an organic compound with the formula (CH2)5C(O)NH. This colourless solid is a lactam (a cyclic amide) of caproic acid. Global demand for this compound is approximately five million tons per year, and the vast majority is used to make Nylon 6 filament, fiber, and plastics. Synthesis and production Caprolactam was first described in the late 1800s when it was prepared by the cyclization of ε-aminocaproic acid, the product of the hydrolysis of caprolactam. World demand for caprolactam was estimated to reach five million tons per year for 2015. 90% of caprolactam produced is used to make filament and fiber, 10% for plastics, and a small amount is used as a chemical intermediate. Due to its commercial significance, many methods have been developed for the production of caprolactam. It was estimated that 90% of all caprolactam is synthesised from cyclohexanone (1), which is first converted to its oxime (2). Treatment of this oxime with acid induces the Beckmann rear ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |