|
Joule–Thomson Effect
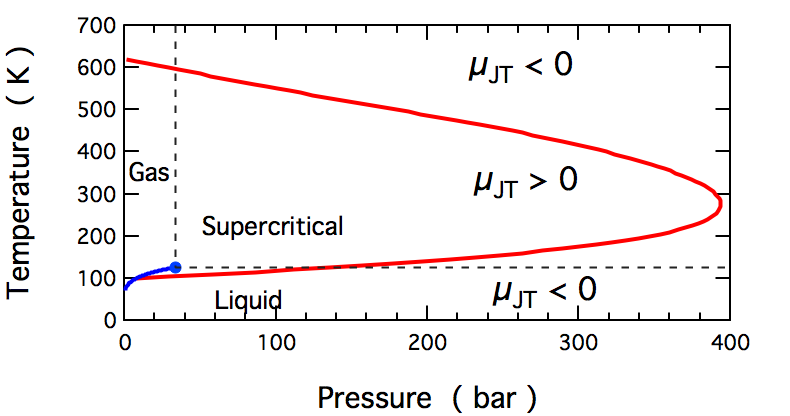
In thermodynamics, the Joule–Thomson effect (also known as the Joule–Kelvin effect or Kelvin–Joule effect) describes the temperature change of a Real gas, ''real'' gas or liquid (as differentiated from an ideal gas) when it is expanding; typically caused by the pressure loss from flow through a expansion valve (steam engine), valve or enthalpy#Throttling, porous plug while keeping it insulated so that Adiabatic process, no heat is exchanged with the environment. This procedure is called a ''throttling process'' or ''Joule–Thomson process''. The effect is purely due to deviation from ideality, as any ideal gas has no JT effect. At room temperature, all gases except hydrogen, helium, and neon cool upon expansion by the Joule–Thomson process when being throttled through an orifice; these three gases rise in temperature when forced through a porous plug at room temperature, but lowers in temperature when already at lower temperatures. Most liquids such as Hydraulics, hydr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thermodynamics
Thermodynamics is a branch of physics that deals with heat, Work (thermodynamics), work, and temperature, and their relation to energy, entropy, and the physical properties of matter and radiation. The behavior of these quantities is governed by the four laws of thermodynamics, which convey a quantitative description using measurable macroscopic physical quantity, physical quantities but may be explained in terms of microscopic constituents by statistical mechanics. Thermodynamics applies to various topics in science and engineering, especially physical chemistry, biochemistry, chemical engineering, and mechanical engineering, as well as other complex fields such as meteorology. Historically, thermodynamics developed out of a desire to increase the thermodynamic efficiency, efficiency of early steam engines, particularly through the work of French physicist Nicolas Léonard Sadi Carnot, Sadi Carnot (1824) who believed that engine efficiency was the key that could help France win ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thermal Imaging Camera
A thermal imaging camera (colloquially known as a TIC) is a type of the thermographic camera used in firefighting. By rendering infrared radiation as visible light, such cameras allow firefighters to see areas of heat through smoke, darkness, or heat-permeable barriers. Thermal imaging cameras are typically handheld, but may be integrated with other pieces of equipment such as helmets and SCBAs. They are constructed using heat- and water-resistant housings, and ruggedized to withstand the hazards of fireground operations, often meeting the requirements of NFPA 1801, ''Standard on Thermal Imagers for the Fire Service''. While they are expensive pieces of equipment, their popularity and adoption by firefighters in the United States is increasing markedly due to the increased availability of government equipment grants following the September 11 attacks in 2001. Thermal imaging cameras pick up body heat, and they are normally used in cases where people are trapped where rescuers ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Internal Energy
The internal energy of a thermodynamic system is the energy of the system as a state function, measured as the quantity of energy necessary to bring the system from its standard internal state to its present internal state of interest, accounting for the gains and losses of energy due to changes in its internal state, including such quantities as magnetization. It excludes the kinetic energy of motion of the system as a whole and the potential energy of position of the system as a whole, with respect to its surroundings and external force fields. It includes the thermal energy, ''i.e.'', the constituent particles' kinetic energies of motion relative to the motion of the system as a whole. Without a thermodynamic process, the internal energy of an isolated system cannot change, as expressed in the law of conservation of energy, a foundation of the first law of thermodynamics. The notion has been introduced to describe the systems characterized by temperature variations, te ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mechanical Work
In science, work is the energy transferred to or from an object via the application of force along a displacement. In its simplest form, for a constant force aligned with the direction of motion, the work equals the product of the force strength and the distance traveled. A force is said to do ''positive work'' if it has a component in the direction of the displacement of the point of application. A force does ''negative work'' if it has a component opposite to the direction of the displacement at the point of application of the force. For example, when a ball is held above the ground and then dropped, the work done by the gravitational force on the ball as it falls is positive, and is equal to the weight of the ball (a force) multiplied by the distance to the ground (a displacement). If the ball is thrown upwards, the work done by the gravitational force is negative, and is equal to the weight multiplied by the displacement in the upwards direction. Both force and displace ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isentropic
An isentropic process is an idealized thermodynamic process that is both adiabatic and reversible. The work transfers of the system are frictionless, and there is no net transfer of heat or matter. Such an idealized process is useful in engineering as a model of and basis of comparison for real processes. This process is idealized because reversible processes do not occur in reality; thinking of a process as both adiabatic and reversible would show that the initial and final entropies are the same, thus, the reason it is called isentropic (entropy does not change). Thermodynamic processes are named based on the effect they would have on the system (ex. isovolumetric: constant volume, isenthalpic: constant enthalpy). Even though in reality it is not necessarily possible to carry out an isentropic process, some may be approximated as such. The word "isentropic" derives from the process being one in which the entropy of the system remains unchanged. In addition to a process wh ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thermodynamic Equilibrium
Thermodynamic equilibrium is a notion of thermodynamics with axiomatic status referring to an internal state of a single thermodynamic system, or a relation between several thermodynamic systems connected by more or less permeable or impermeable walls. In thermodynamic equilibrium, there are no net macroscopic flows of mass nor of energy within a system or between systems. In a system that is in its own state of internal thermodynamic equilibrium, not only is there an absence of macroscopic change, but there is an “absence of any ''tendency'' toward change on a macroscopic scale.” Systems in mutual thermodynamic equilibrium are simultaneously in mutual thermal, mechanical, chemical, and radiative equilibria. Systems can be in one kind of mutual equilibrium, while not in others. In thermodynamic equilibrium, all kinds of equilibrium hold at once and indefinitely, unless disturbed by a thermodynamic operation. In a macroscopic equilibrium, perfectly or almost perfectly ba ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Reversible Process (thermodynamics)
In thermodynamics, a reversible process is a process, involving a system and its surroundings, whose direction can be reversed by infinitesimal changes in some properties of the surroundings, such as pressure or temperature. Throughout an entire reversible process, the system is in thermodynamic equilibrium, both physical and chemical, and ''nearly'' in pressure and temperature equilibrium with its surroundings. This prevents unbalanced forces and acceleration of moving system boundaries, which in turn avoids friction and other dissipation. To maintain equilibrium, reversible processes are extremely slow ( ''quasistatic''). The process must occur slowly enough that after some small change in a thermodynamic parameter, the physical processes in the system have enough time for the other parameters to self-adjust to match the new, changed parameter value. For example, if a container of water has sat in a room long enough to match the steady temperature of the surrounding air, for ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Adiabatic Process
An adiabatic process (''adiabatic'' ) is a type of thermodynamic process that occurs without transferring heat between the thermodynamic system and its Environment (systems), environment. Unlike an isothermal process, an adiabatic process transfers energy to the surroundings only as Work (thermodynamics), work and/or mass flow.. A translation may be founhere. Also a mostly reliabltranslation is to be foundin As a key concept in thermodynamics, the adiabatic process supports the theory that explains the first law of thermodynamics. The opposite term to "adiabatic" is ''diabatic''. Some chemical and physical processes occur too rapidly for energy to enter or leave the system as heat, allowing a convenient "adiabatic approximation".Bailyn, M. (1994), pp. 52–53. For example, the adiabatic flame temperature uses this approximation to calculate the upper limit of fire, flame temperature by assuming combustion loses no heat to its surroundings. In meteorology, adiabatic expansion an ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Vacuum
A vacuum (: vacuums or vacua) is space devoid of matter. The word is derived from the Latin adjective (neuter ) meaning "vacant" or "void". An approximation to such vacuum is a region with a gaseous pressure much less than atmospheric pressure. Physicists often discuss ideal test results that would occur in a ''perfect'' vacuum, which they sometimes simply call "vacuum" or free space, and use the term partial vacuum to refer to an actual imperfect vacuum as one might have in a laboratory or in space. In engineering and applied physics on the other hand, vacuum refers to any space in which the pressure is considerably lower than atmospheric pressure. The Latin term ''in vacuo'' is used to describe an object that is surrounded by a vacuum. The ''quality'' of a partial vacuum refers to how closely it approaches a perfect vacuum. Other things equal, lower gas pressure means higher-quality vacuum. For example, a typical vacuum cleaner produces enough suction to reduce air pressur ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
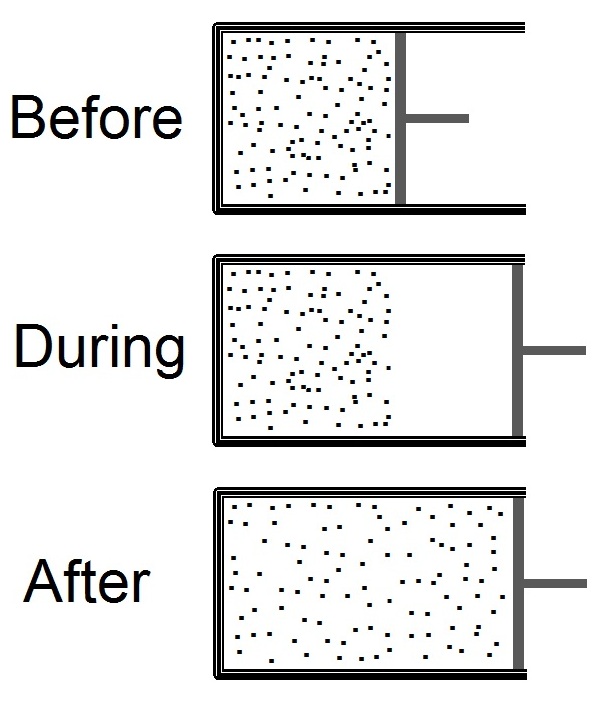
Joule Expansion
The Joule expansion (a subset of free expansion) is an irreversible process in thermodynamics in which a volume of gas is kept in one side of a thermally isolated container (via a small partition), with the other side of the container being evacuated. The partition between the two parts of the container is then opened, and the gas fills the whole container. The Joule expansion, treated as a thought experiment involving ideal gases, is a useful exercise in classical thermodynamics. It provides a convenient example for calculating changes in thermodynamic quantities, including the resulting increase in entropy of the universe (entropy production) that results from this inherently irreversible process. An actual Joule expansion experiment necessarily involves real gases; the temperature change in such a process provides a measure of intermolecular forces. This type of expansion is named after James Prescott Joule who used this expansion, in 1845, in his study for the mechanical e ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
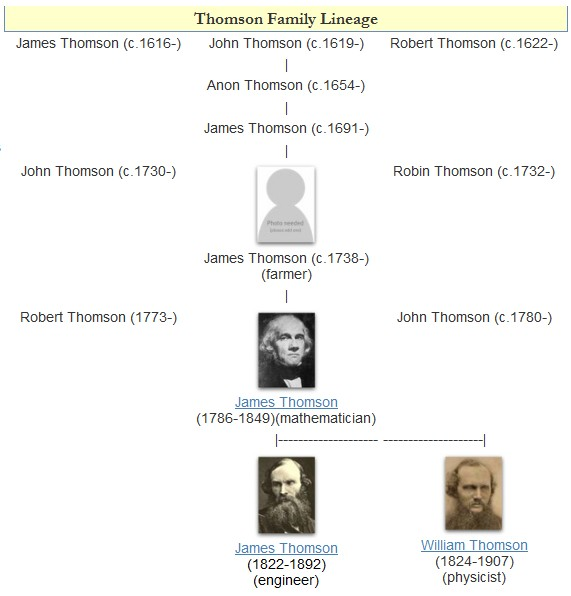
William Thomson, 1st Baron Kelvin
William Thomson, 1st Baron Kelvin (26 June 182417 December 1907), was a British mathematician, Mathematical physics, mathematical physicist and engineer. Born in Belfast, he was the Professor of Natural Philosophy (Glasgow), professor of Natural Philosophy at the University of Glasgow for 53 years, where he undertook significant research on the mathematical analysis of electricity, was instrumental in the formulation of the first and second laws of thermodynamics, and contributed significantly to unifying physics, which was then in its infancy of development as an emerging academic discipline. He received the Royal Society's Copley Medal in 1883 and served as its President of the Royal Society, president from 1890 to 1895. In 1892, he became the first scientist to be elevated to the House of Lords. Absolute temperatures are stated in units of kelvin in Lord Kelvin's honour. While the existence of a coldest possible temperature, absolute zero, was known before his work, Kelvin d ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
James Prescott Joule
James Prescott Joule (; 24 December 1818 11 October 1889) was an English physicist. Joule studied the nature of heat and discovered its relationship to mechanical work. This led to the law of conservation of energy, which in turn led to the development of the first law of thermodynamics. The SI unit of energy, the joule (J), is named after him. He worked with Lord Kelvin to develop an absolute thermodynamic temperature scale, which came to be called the Kelvin scale. Joule also made observations of magnetostriction, and he found the relationship between the current through a resistor and the heat dissipated, which is also called Joule's first law. His experiments about energy transformations were first published in 1843. Early years James Joule was born in 1818, the son of Benjamin Joule (1784–1858), a wealthy brewer, and his wife, Alice Prescott, on New Bailey Street in Salford. Joule was tutored as a young man by the famous scientist John Dalton and was strongly in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |