|
Jeffrey R. Long
Jeffrey R. Long is a professor of chemistry at the University of California, Berkeley known for his work in Metal–organic framework, metal−organic frameworks and Single-molecule magnet, molecular magnetism. He was elected to the American Academy of Arts and Sciences in 2019 and is the 2019 F. Albert Cotton Award recipient. His research interests include: the synthesis of inorganic clusters and porous materials, investigating the electronic and magnetic properties of inorganic materials; metal-organic frameworks, and gas storage/capture. Early life and education Jeffrey Long was born in Rolla, Missouri on May 15, 1969. He is the son of Gary J. Long, Prof. Emeritus of Chemistry at the Missouri University of Science and Technology, an expert in Mössbauer spectroscopy. He received his Bachelor of Arts, Bachelors of Arts from Cornell University in Chemistry (''Latin honors, summa cum laude'') and Mathematics (''Latin honors, cum laude'') in 1991. While an undergraduate student, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Inorganic Chemistry
Inorganic chemistry deals with synthesis and behavior of inorganic and organometallic compounds. This field covers chemical compounds that are not carbon-based, which are the subjects of organic chemistry. The distinction between the two disciplines is far from absolute, as there is much overlap in the subdiscipline of organometallic chemistry. It has applications in every aspect of the chemical industry, including catalysis, materials science, pigments, surfactants, coatings, medications, fuels, and agriculture. Key concepts Many inorganic compounds are ionic compounds, consisting of cations and anions joined by ionic bonding. Examples of salts (which are ionic compounds) are magnesium chloride MgCl2, which consists of magnesium cations Mg2+ and chloride anions Cl−; or sodium oxide Na2O, which consists of sodium cations Na+ and oxide anions O2−. In any salt, the proportions of the ions are such that the electric charges cancel out, so that the bulk compound is e ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
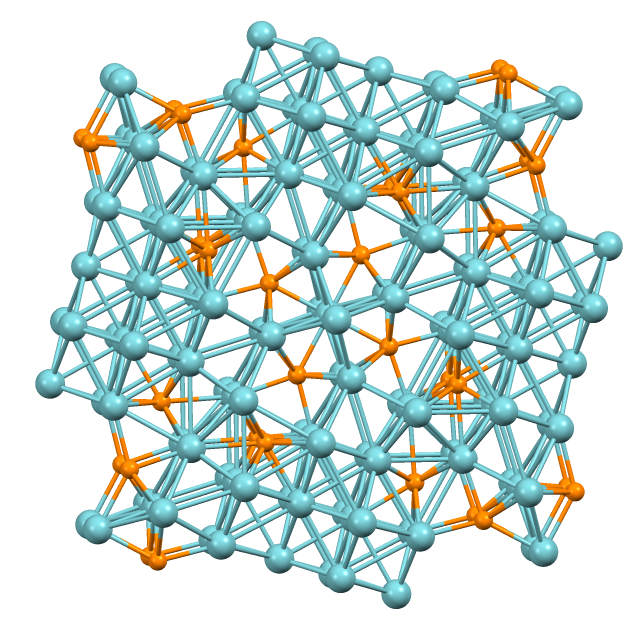
Metal–organic Framework
Metal–organic frameworks (MOFs) are a class of compounds consisting of metal ions or cluster compound, clusters coordinated to organic compound, organic ligands to form one-, two-, or three-dimensional structures. The organic ligands included are sometimes referred to as "struts" or "linkers", one example being 1,4-benzenedicarboxylic acid (BDC). More formally, a metal–organic framework is a crystalline material with organic ligands containing potential voids. In most cases for MOFs, the pores are stable during the elimination of the guest molecules (often solvents) and could be refilled with other compounds. Because of this property, MOFs are of interest for the storage of gases such as hydrogen and carbon dioxide. Other possible applications of MOFs are in gas purification, in gas separation, in Groundwater remediation, water remediation, in catalysis, as conducting solids and as supercapacitors. The synthesis and properties of MOFs constitute the primary focus of the disci ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cobalt
Cobalt is a chemical element with the symbol Co and atomic number 27. As with nickel, cobalt is found in the Earth's crust only in a chemically combined form, save for small deposits found in alloys of natural meteoric iron. The free element, produced by reductive smelting, is a hard, lustrous, silver-gray metal. Cobalt-based blue pigments ( cobalt blue) have been used since ancient times for jewelry and paints, and to impart a distinctive blue tint to glass, but the color was for a long time thought to be due to the known metal bismuth. Miners had long used the name ''kobold ore'' (German for ''goblin ore'') for some of the blue-pigment-producing minerals; they were so named because they were poor in known metals, and gave poisonous arsenic-containing fumes when smelted. In 1735, such ores were found to be reducible to a new metal (the first discovered since ancient times), and this was ultimately named for the ''kobold''. Today, some cobalt is produced specifically from one of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Magnetism
Magnetism is the class of physical attributes that are mediated by a magnetic field, which refers to the capacity to induce attractive and repulsive phenomena in other entities. Electric currents and the magnetic moments of elementary particles give rise to a magnetic field, which acts on other currents and magnetic moments. Magnetism is one aspect of the combined phenomena of electromagnetism. The most familiar effects occur in ferromagnetic materials, which are strongly attracted by magnetic fields and can be magnetized to become permanent magnets, producing magnetic fields themselves. Demagnetizing a magnet is also possible. Only a few substances are ferromagnetic; the most common ones are iron, cobalt, and nickel and their alloys. The rare-earth metals neodymium and samarium are less common examples. The prefix ' refers to iron because permanent magnetism was first observed in lodestone, a form of natural iron ore called magnetite, Fe3O4. All substances exhibit some type of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Prussian Blue
Prussian blue (also known as Berlin blue, Brandenburg blue or, in painting, Parisian or Paris blue) is a dark blue pigment produced by oxidation of ferrous ferrocyanide salts. It has the chemical formula Fe CN)">Cyanide.html" ;"title="e(Cyanide">CN) Turnbull's blue is chemically identical, but is made from different reagents, and its slightly different color stems from different impurities and particle sizes. Prussian blue was the first modern synthetic pigment. It is prepared as a very fine colloidal dispersion, because the compound is not soluble in water. It contains variable amounts of other ions and its appearance depends sensitively on the size of the colloidal particles. The pigment is used in paints, and it is the traditional "blue" in blueprints, and became prominent in 19th-century () Japanese woodblock prints. In medicine, orally administered Prussian blue is used as an antidote for certain kinds of heavy metal poisoning, e.g., by thallium(I) and radioactive is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chalcogenide
: 220px, Cadmium sulfide, a prototypical metal chalcogenide, is used as a yellow pigment. A chalcogenide is a chemical compound consisting of at least one chalcogen anion and at least one more electropositive element. Although all group 16 elements of the periodic table are defined as chalcogens, the term chalcogenide is more commonly reserved for sulfides, selenides, tellurides, and polonides, rather than oxides. Many metal ores exist as chalcogenides. Photoconductive chalcogenide glasses are used in xerography. Some pigments and catalysts are also based on chalcogenides. The metal dichalcogenide MoS2 is a common solid lubricant. Alkali metal and alkaline earth chalcogenides Alkali metal and alkaline earth monochalcogenides are salt-like, being colourless and often water-soluble. The sulfides tend to undergo hydrolysis to form derivatives containing bisulfide (SH−) anions. The alkali metal chalcogenides often crystallize with the antifluorite structure and the alkaline earth s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Transition Metal
In chemistry, a transition metal (or transition element) is a chemical element in the d-block of the periodic table (groups 3 to 12), though the elements of group 12 (and less often group 3) are sometimes excluded. They are the elements that can use d orbitals as valence orbitals to form chemical bonds. The lanthanide and actinide elements (the f-block) are called inner transition metals and are sometimes considered to be transition metals as well. Since they are metals, they are lustrous and have good electrical and thermal conductivity. Most (with the exception of group 11 and group 12) are hard and strong, and have high melting and boiling temperatures. They form compounds in any of two or more different oxidation states and bind to a variety of ligands to form coordination complexes that are often coloured. They form many useful alloys and are often employed as catalysts in elemental form or in compounds such as coordination complexes and oxides. Most are strongly param ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electronic Band Structure
In solid-state physics, the electronic band structure (or simply band structure) of a solid describes the range of energy levels that electrons may have within it, as well as the ranges of energy that they may not have (called ''band gaps'' or ''forbidden bands''). Band theory derives these bands and band gaps by examining the allowed quantum mechanical wave functions for an electron in a large, periodic lattice of atoms or molecules. Band theory has been successfully used to explain many physical properties of solids, such as electrical resistivity and optical absorption, and forms the foundation of the understanding of all solid-state devices (transistors, solar cells, etc.). Why bands and band gaps occur The electrons of a single, isolated atom occupy atomic orbitals each of which has a discrete energy level. When two or more atoms join together to form a molecule, their atomic orbitals overlap and hybridize. Similarly, if a large number ''N'' of identical atoms come ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Molecular Orbital Theory
In chemistry, molecular orbital theory (MO theory or MOT) is a method for describing the electronic structure of molecules using quantum mechanics. It was proposed early in the 20th century. In molecular orbital theory, electrons in a molecule are not assigned to individual chemical bonds between atoms, but are treated as moving under the influence of the atomic nuclei in the whole molecule. Quantum mechanics describes the spatial and energetic properties of electrons as molecular orbitals that surround two or more atoms in a molecule and contain valence electrons between atoms. Molecular orbital theory revolutionized the study of chemical bonding by approximating the states of bonded electrons—the molecular orbitals—as linear combinations of atomic orbitals (LCAO). These approximations are made by applying the density functional theory (DFT) or Hartree–Fock (HF) models to the Schrödinger equation. Molecular orbital theory and valence bond theory are the foundational ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Latin Honors
Latin honors are a system of Latin phrases used in some colleges and universities to indicate the level of distinction with which an academic degree has been earned. The system is primarily used in the United States. It is also used in some Southeastern Asian countries with European colonial history, such as Indonesia and the Philippines, although sometimes translations of these phrases are used instead of the Latin originals. The honors distinction should not be confused with the honors degrees offered in some countries, or with honorary degrees. The system usually has three levels of honor: ''cum laude'', ''magna cum laude'', and ''summa cum laude''. Generally, a college or university's regulations set out definite criteria a student must meet to obtain a given honor. For example, the student might be required to achieve a specific grade point average, submit an honors thesis for evaluation, be part of an honors program, or graduate early. Each school sets its own standards. S ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bachelor Of Arts
Bachelor of arts (BA or AB; from the Latin ', ', or ') is a bachelor's degree awarded for an undergraduate program in the arts, or, in some cases, other disciplines. A Bachelor of Arts degree course is generally completed in three or four years, depending on the country and institution. * Degree attainment typically takes four years in Afghanistan, Armenia, Azerbaijan, Bangladesh, Brazil, Brunei, China, Egypt, Ghana, Greece, Georgia, Hong Kong, Indonesia, Iran, Iraq, Ireland, Japan, Kazakhstan, Kenya, Kuwait, Latvia, Lebanon, Lithuania, Mexico, Malaysia, Mongolia, Myanmar, Nepal, Netherlands, Nigeria, Pakistan, the Philippines, Qatar, Russia, Saudi Arabia, Scotland, Serbia, South Korea, Spain, Sri Lanka, Taiwan, Thailand, Turkey, Ukraine, the United States and Zambia. * Degree attainment typically takes three years in Albania, Australia, Bosnia and Herzegovina, the Caribbean, Iceland, India, Israel, Italy, New Zealand, Norway, South Africa, Switzerland, the Canadian province of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mössbauer Spectroscopy
Mössbauer spectroscopy is a spectroscopic technique based on the Mössbauer effect. This effect, discovered by Rudolf Mössbauer (sometimes written "Moessbauer", German: "Mößbauer") in 1958, consists of the nearly recoil-free emission and absorption of nuclear gamma rays in solids. The consequent nuclear spectroscopy method is exquisitely sensitive to small changes in the chemical environment of certain nuclei. Typically, three types of nuclear interactions may be observed: the isomer shift due to differences in nearby electron densities (also called the chemical shift in older literature), quadrupole splitting due to atomic-scale electric field gradients; and magnetic Zeeman splitting due to non-nuclear magnetic fields. Due to the high energy and extremely narrow line widths of nuclear gamma rays, Mössbauer spectroscopy is a highly sensitive technique in terms of energy (and hence frequency) resolution, capable of detecting changes of just a few parts in 1011. It is a me ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |