|
JWH-164
JWH-164 is a synthetic cannabinoid receptor agonist from the naphthoylindole family. It has approximately equal affinity for the CB1 and CB2 receptors, with a Ki of 6.6 nM at CB1 and 6.9 nM at CB2. JWH-164 is a positional isomer of the related compound JWH-081, but with a methoxy group at the 7-position of the naphthyl ring, rather than the 4-position as in JWH-081. Its potency is intermediate between that of JWH-081 and its ring unsubstituted derivative JWH-018, demonstrating that substitution of the naphthyl 7-position can also result in increased cannabinoid receptor binding affinity. In the United States, all CB1 all receptor agonists of the 3-(1-naphthoyl)indole class, including JWH-164, are Schedule I Controlled Substance This is the list of Schedule I drugs as defined by the United States Controlled Substances Act. 21 CFRbr>1308.11(CSA Sched I) with changes through (Oct 18, 2012). Retrieved September 6, 2013. The following findings are required for drugs to ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
JWH Cannabinoids
The John W. Huffman research group at Clemson University synthesized over 450 cannabinoids. Some of those are: [Baidu] |
JWH-081
JWH-081 is an analgesic chemical from the naphthoylindole family, which acts as a cannabinoid agonist at both the CB1 and CB2 receptors. With a Ki of 1.2nM it is fairly selective for the CB1 subtype, its affinity at this subtype is measured at approximately 10x the affinity at CB2(12.4nM). It was discovered by and named after John W. Huffman. JWH-081 may be neurotoxic to animals when administered in high doses. Legal status In the United States, JWH-081 is a Schedule I Controlled Substance. As of October 2015, JWH-081 is a controlled substance in China. See also *JWH-018 *JWH-098 *JWH-164 *JWH-198 *JWH-210 JWH-210 is an analgesic chemical from the naphthoylindole family, which acts as a potent cannabinoid agonist at both the CB1 and CB2 receptors, with Ki values of 0.46 nM at CB1 and 0.69 nM at CB2. It is one of the most potent 4-substi ... References Designer drugs JWH cannabinoids Naphthoylindoles Phenol ethers CB1 receptor agonists {{canna ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
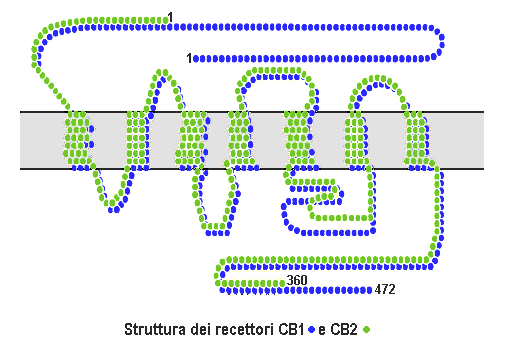
Cannabinoid Receptor
Cannabinoid receptors, located throughout the body, are part of the endocannabinoid system a class of cell membrane receptors in the G protein-coupled receptor superfamily. As is typical of G protein-coupled receptors, the cannabinoid receptors contain seven transmembrane spanning domains. Cannabinoid receptors are activated by three major groups of ligands: endocannabinoids; plant cannabinoids (such as Tetrahydrocannabinol, produced by the cannabis plant); and synthetic cannabinoids (such as HU-210). All of the endocannabinoids and phytocannabinoids (plant based cannabinoids) are lipophilic. There are two known subtypes of cannabinoid receptors, termed CB1 and CB2. The CB1 receptor is expressed mainly in the brain (central nervous system or "CNS"), but also in the lungs, liver and kidneys. The CB2 receptor is expressed mainly in the immune system, in hematopoietic cells, and in parts of the brain. The protein sequences of CB1 and CB2 receptors are about 44% similar. When ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Agonist
An agonist is a chemical that activates a receptor to produce a biological response. Receptors are cellular proteins whose activation causes the cell to modify what it is currently doing. In contrast, an antagonist blocks the action of the agonist, while an inverse agonist causes an action opposite to that of the agonist. Etymology From the Greek αγωνιστής (agōnistēs), contestant; champion; rival < αγων (agōn), contest, combat; exertion, struggle < αγω (agō), I lead, lead towards, conduct; drive Types of agonists can be activated by either endogenous agonists (such as |
Cannabinoid Receptor 1
Cannabinoid receptor type 1 (CB1), also known as cannabinoid receptor 1, is a G protein-coupled cannabinoid receptor that in humans is encoded by the ''CNR1'' gene. The human CB1 receptor is expressed in the peripheral nervous system and central nervous system. It is activated by: endocannabinoids, a group of retrograde neurotransmitters that include anandamide and 2-arachidonoylglycerol (2-AG); plant phytocannabinoids, such as the compound THC which is an active ingredient of the psychoactive drug cannabis; and, synthetic analogs of THC. CB1 is antagonized by the phytocannabinoid tetrahydrocannabivarin (THCV). The primary endogenous agonist of the human CB1 receptor is anandamide. Structure The CB1 receptor shares the structure characteristic of all G-protein-coupled receptors, possessing seven transmembrane domains connected by three extracellular and three intracellular loops, an extracellular N-terminal tail, and an intracellular C-terminal tail. The receptor may exist ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cannabinoid Receptor 2
The cannabinoid receptor type 2, abbreviated as CB2, is a G protein-coupled receptor from the cannabinoid receptor family that in humans is encoded by the ''CNR2'' gene. It is closely related to the cannabinoid receptor type 1 (CB1), which is largely responsible for the efficacy of endocannabinoid-mediated presynaptic-inhibition, the psychoactive properties of tetrahydrocannabinol (THC), the active agent in cannabis, and other phytocannabinoids (plant cannabinoids). The principal endogenous ligand for the CB2 receptor is 2-Arachidonoylglycerol (2-AG). CB2 was cloned in 1993 by a research group from Cambridge looking for a second cannabinoid receptor that could explain the pharmacological properties of tetrahydrocannabinol. The receptor was identified among cDNAs based on its similarity in amino-acid sequence to the cannabinoid receptor type 1 (CB1) receptor, discovered in 1990. The discovery of this receptor helped provide a molecular explanation for the established effects of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dissociation Constant
In chemistry, biochemistry, and pharmacology, a dissociation constant (K_D) is a specific type of equilibrium constant that measures the propensity of a larger object to separate (dissociate) reversibly into smaller components, as when a complex falls apart into its component molecules, or when a salt splits up into its component ions. The dissociation constant is the inverse of the association constant. In the special case of salts, the dissociation constant can also be called an ionization constant. For a general reaction: : A_\mathit B_\mathit \mathit A + \mathit B in which a complex \ce_x \ce_y breaks down into ''x'' A subunits and ''y'' B subunits, the dissociation constant is defined as : K_D = \frac where and ''x'' B''y''are the equilibrium concentrations of A, B, and the complex A''x'' B''y'', respectively. One reason for the popularity of the dissociation constant in biochemistry and pharmacology is that in the frequently encount ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Positional Isomer
In chemistry, a structural isomer (or constitutional isomer in the IUPAC nomenclature) of a compound is another compound whose molecule has the same number of atoms of each element, but with logically distinct bonds between them. The term metamer was formerly used for the same concept. For example, butanol , methyl propyl ether , and diethyl ether have the same molecular formula but are three distinct structural isomers. The concept applies also to polyatomic ions with the same total charge. A classical example is the cyanate ion and the fulminate ion . It is also extended to ionic compounds, so that (for example) ammonium cyanate and urea are considered structural isomers,William F. Bynum, E. Janet Browne, Roy Porter (2014): ''Dictionary of the History of Science''. 530 pages. and so are methylammonium formate and ammonium acetate . Structural isomerism is the most radical type of isomerism. It is opposed to stereoisomerism, in which the atoms and bonding scheme ar ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Methoxy
In organic chemistry, a methoxy group is the functional group consisting of a methyl group bound to oxygen. This alkoxy group has the formula . On a benzene ring, the Hammett equation classifies a methoxy substituent at the ''para'' position as an electron-donating group, but as an electron-withdrawing group if at the ''meta'' position. At the ''ortho'' position, steric effects are likely to cause a significant alteration in the Hammett equation prediction which otherwise follows the same trend as that of the ''para'' position. Occurrence The simplest of methoxy compounds are methanol and dimethyl ether. Other methoxy ethers include anisole and vanillin. Many alkoxides contain methoxy groups, e.g. tetramethyl orthosilicate and titanium methoxide. Such compounds are often classified as methoxides. Esters with a methoxy group can be referred to as methyl esters, and the —COOCH3 substituent is called a methoxycarbonyl. Biosynthesis In nature, methoxy groups are found on nucleosi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Naphthyl
Naphthalene is an organic compound with formula . It is the simplest polycyclic aromatic hydrocarbon, and is a white crystalline solid with a characteristic odor that is detectable at concentrations as low as 0.08 ppm by mass. As an aromatic hydrocarbon, naphthalene's structure consists of a fused pair of benzene rings. It is best known as the main ingredient of traditional mothballs. History In the early 1820s, two separate reports described a white solid with a pungent odor derived from the distillation of coal tar. In 1821, John Kidd cited these two disclosures and then described many of this substance's properties and the means of its production. He proposed the name ''naphthaline'', as it had been derived from a kind of naphtha (a broad term encompassing any volatile, flammable liquid hydrocarbon mixture, including coal tar). Naphthalene's chemical formula was determined by Michael Faraday in 1826. The structure of two fused benzene rings was proposed by Emil Erlenmey ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
JWH-018
JWH-018 (1-pentyl-3-(1-naphthoyl)indole, NA-PIMO or AM-678) is an analgesic chemical from the naphthoylindole family that acts as a full agonist at both the CB1 and CB2 cannabinoid receptors, with some selectivity for CB2. It produces effects in animals similar to those of tetrahydrocannabinol (THC), a cannabinoid naturally present in cannabis, leading to its use in synthetic cannabis products that in some countries are sold legally as "incense blends". As a full agonist at both the CB1 and CB2 cannabinoid receptors, this chemical compound is classified as an analgesic medication. The analgesic effects of cannabinoid ligands, mediated by CB1 receptors are well established in treatment of neuropathic pain, as well as cancer pain and arthritis. These compounds work by mimicking the body's naturally-produced endocannabinoid hormones such as 2-AG and anandamide (AEA), which are biologically active and can exacerbate or inhibit nerve signaling. As the cause is poorly understood in chr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Schedule I Controlled Substance
This is the list of Schedule I drugs as defined by the United States Controlled Substances Act. 21 CFRbr>1308.11(CSA Sched I) with changes through (Oct 18, 2012). Retrieved September 6, 2013. The following findings are required for drugs to be placed in this schedule: # The drug or other substance has a high potential for abuse. # The drug or other substance has no currently accepted medical use in treatment in the United States. # There is a lack of accepted safety for use of the drug or other substance under medical supervision. Except as specifically authorized, it is illegal for any person: # to manufacture, distribute, or dispense, or possess with intent to manufacture, distribute, or dispense, a controlled substance; or # to create, distribute, dispense, or possess with intent to distribute or dispense, a counterfeit substance. Additional substances are added to the list by the Secretary of Health and Human Services pursuant to 21 CFR 1308.49. [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |