|
JWH-015
JWH-015 is a chemical from the naphthoylindole family that acts as a subtype-selective cannabinoid agonist. Its affinity for CB2 receptors is 13.8 nM, while its affinity for CB1 is 383 nM, meaning that it binds almost 28 times more strongly to CB2 than to CB1. However, it still displays some CB1 activity, and in some model systems can be very potent and efficacious at activating CB1 receptors, and therefore it is not as selective as newer drugs such as JWH-133. It has been shown to possess immunomodulatory effects, and CB2 agonists may be useful in the treatment of pain and inflammation. It was discovered and named after John W. Huffman. Metabolism JWH-015 has been shown ''in vitro'' to be metabolized primarily by hydroxylation and ''N''- dealkylation, and also by epoxidation of the naphthalene ring, similar to the metabolic pathways seen for other aminoalkylindole cannabinoids such as WIN 55,212-2. Epoxidation of polycyclic aromatic hydrocarbons (see for example benzo(a) ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cannabinoid Receptor Type 2
The cannabinoid receptor type 2, abbreviated as CB2, is a G protein-coupled receptor from the cannabinoid receptor family that in humans is encoded by the ''CNR2'' gene. It is closely related to the cannabinoid receptor type 1 (CB1), which is largely responsible for the efficacy of endocannabinoid-mediated presynaptic-inhibition, the psychoactive properties of tetrahydrocannabinol (THC), the active agent in cannabis, and other phytocannabinoids (plant cannabinoids). The principal endogenous ligand for the CB2 receptor is 2-Arachidonoylglycerol (2-AG). CB2 was cloned in 1993 by a research group from Cambridge looking for a second cannabinoid receptor that could explain the pharmacological properties of tetrahydrocannabinol. The receptor was identified among cDNAs based on its similarity in amino-acid sequence to the cannabinoid receptor type 1 (CB1) receptor, discovered in 1990. The discovery of this receptor helped provide a molecular explanation for the established effects of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
JWH Cannabinoids
The John W. Huffman research group at Clemson University synthesized over 450 cannabinoids. Some of those are: [Baidu] |
Cannabinoid Receptor Type 1
Cannabinoid receptor type 1 (CB1), also known as cannabinoid receptor 1, is a G protein-coupled cannabinoid receptor that in humans is encoded by the ''CNR1'' gene. The human CB1 receptor is expressed in the peripheral nervous system and central nervous system. It is activated by: endocannabinoids, a group of retrograde neurotransmitters that include anandamide and 2-arachidonoylglycerol (2-AG); plant phytocannabinoids, such as the compound THC which is an active ingredient of the psychoactive drug cannabis; and, synthetic analogs of THC. CB1 is antagonized by the phytocannabinoid tetrahydrocannabivarin (THCV). The primary endogenous agonist of the human CB1 receptor is anandamide. Structure The CB1 receptor shares the structure characteristic of all G-protein-coupled receptors, possessing seven transmembrane domains connected by three extracellular and three intracellular loops, an extracellular N-terminal tail, and an intracellular C-terminal tail. The receptor may exist ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Naphthoylindole
Naphthoylindoles are a class of synthetic cannabinoids. See also * Structural scheduling of synthetic cannabinoids To combat the illicit synthetic cannabinoid industry many jurisdictions have created a system to control these cannabinoids through their general (or Markush) structure as opposed to their specific identity. In this way new analogs are already cont ... References {{reflist ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
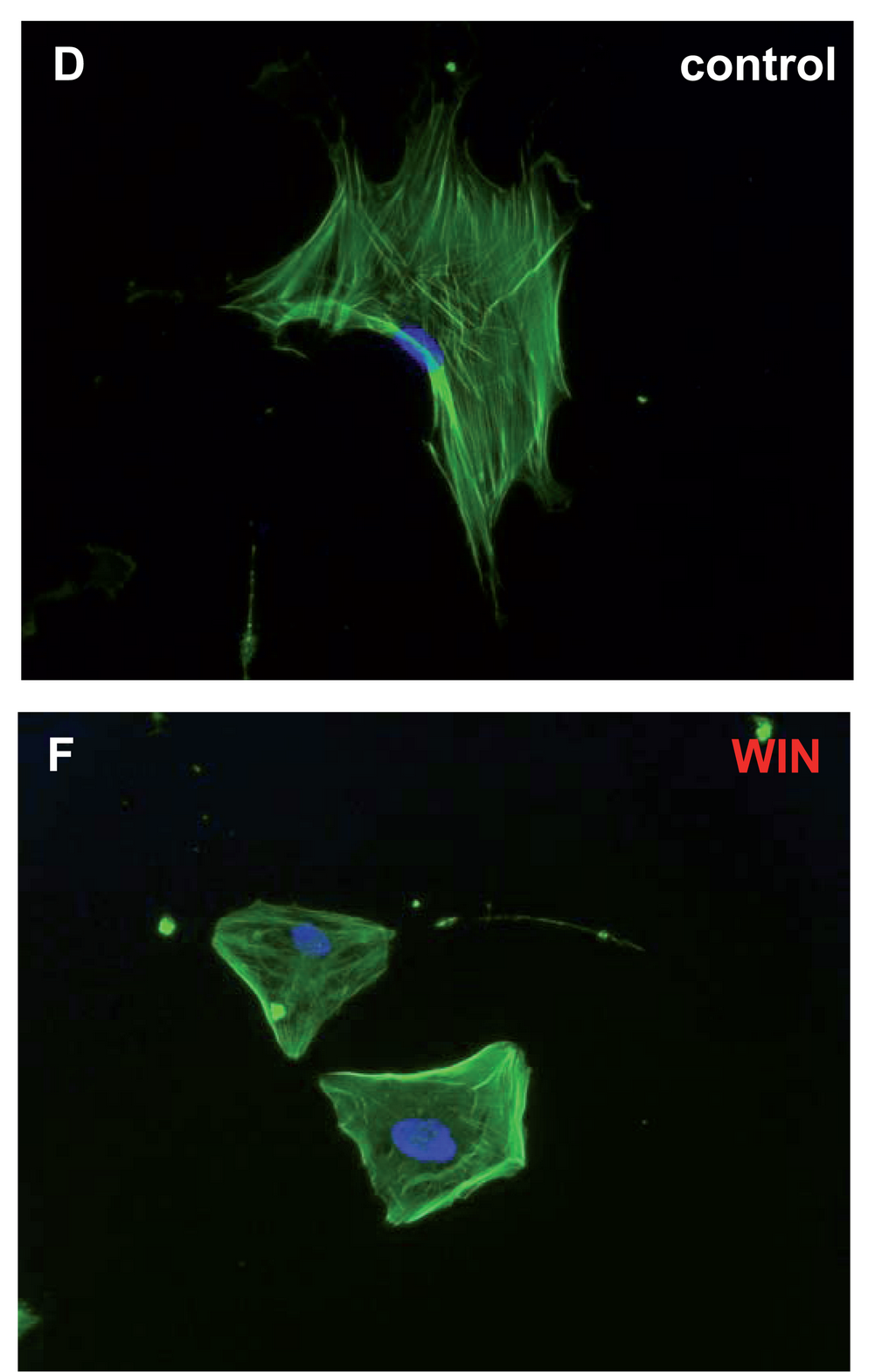
WIN 55,212-2
WIN 55,212-2 is a chemical described as an aminoalkylindole derivative, which produces effects similar to those of cannabinoids such as tetrahydrocannabinol (THC) but has an entirely different chemical structure. WIN 55,212-2 is a potent cannabinoid receptor agonist that has been found to be a potent analgesic in a rat model of neuropathic pain. It activates p42 and p44 MAP kinase via receptor-mediated signaling. At 5 μM WIN 55,212-2 inhibits ATP production in sperm in a CB1 receptor-dependent fashion. WIN 55,212-2, along with HU-210 and JWH-133, may prevent the inflammation caused by amyloid beta proteins involved in Alzheimer's disease, in addition to preventing cognitive impairment and loss of neuronal markers. This anti-inflammatory action is induced through agonist action at cannabinoid receptors, which prevents microglial activation that elicits the inflammation. WIN 55,212-2 is a full agonist at the CB1 cannabinoid receptor ( ''K''i = 1.9 nM) and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Designer Drugs
A designer drug is a structural or functional analog of a controlled substance that has been designed to mimic the pharmacological effects of the original drug, while avoiding classification as illegal and/or detection in standard drug tests. Designer drugs include psychoactive substances that have been designated by the European Union as new psychoactive substances (NPS) as well as analogs of performance-enhancing drugs such as designer steroids. Some of these were originally synthesized by academic or industrial researchers in an effort to discover more potent derivatives with fewer side effects, and shorter duration (and possibly also because it is easier to apply for patents for new molecules) and were later co-opted for recreational use. Other designer drugs were prepared for the first time in clandestine laboratories. Because the efficacy and safety of these substances have not been thoroughly evaluated in animal and human trials, the use of some of these drugs may result i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Naphthoylindoles
Naphthoylindoles are a class of synthetic cannabinoids. See also * Structural scheduling of synthetic cannabinoids To combat the illicit synthetic cannabinoid industry many jurisdictions have created a system to control these cannabinoids through their general (or Markush) structure as opposed to their specific identity. In this way new analogs are already cont ... References {{reflist ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Schedule I Controlled Substance
This is the list of Schedule I drugs as defined by the United States Controlled Substances Act. 21 CFRbr>1308.11(CSA Sched I) with changes through (Oct 18, 2012). Retrieved September 6, 2013. The following findings are required for drugs to be placed in this schedule: # The drug or other substance has a high potential for abuse. # The drug or other substance has no currently accepted medical use in treatment in the United States. # There is a lack of accepted safety for use of the drug or other substance under medical supervision. Except as specifically authorized, it is illegal for any person: # to manufacture, distribute, or dispense, or possess with intent to manufacture, distribute, or dispense, a controlled substance; or # to create, distribute, dispense, or possess with intent to distribute or dispense, a counterfeit substance. Additional substances are added to the list by the Secretary of Health and Human Services pursuant to 21 CFR 1308.49. [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Apoptosis
Apoptosis (from grc, ἀπόπτωσις, apóptōsis, 'falling off') is a form of programmed cell death that occurs in multicellular organisms. Biochemical events lead to characteristic cell changes (morphology) and death. These changes include blebbing, cell shrinkage, nuclear fragmentation, chromatin condensation, DNA fragmentation, and mRNA decay. The average adult human loses between 50 and 70 billion cells each day due to apoptosis. For an average human child between eight and fourteen years old, approximately twenty to thirty billion cells die per day. In contrast to necrosis, which is a form of traumatic cell death that results from acute cellular injury, apoptosis is a highly regulated and controlled process that confers advantages during an organism's life cycle. For example, the separation of fingers and toes in a developing human embryo occurs because cells between the digits undergo apoptosis. Unlike necrosis, apoptosis produces cell fragments called apoptotic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carcinogenic
A carcinogen is any substance, radionuclide, or radiation that promotes carcinogenesis (the formation of cancer). This may be due to the ability to damage the genome or to the disruption of cellular metabolic processes. Several radioactive substances are considered carcinogens, but their carcinogenic activity is attributed to the radiation, for example gamma rays and alpha particles, which they emit. Common examples of non-radioactive carcinogens are inhaled asbestos, certain dioxins, and tobacco smoke. Although the public generally associates carcinogenicity with synthetic chemicals, it is equally likely to arise from both natural and synthetic substances. Carcinogens are not necessarily immediately toxic; thus, their effect can be insidious. Carcinogens, as mentioned, are agents in the environment capable of contributing to cancer growth. Carcinogens can be categorized into two different types: activation-dependent and activation-independent, and each nature impacts their level ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Benzo(a)pyrene
Benzo 'a''yrene (B''a''P or B ) is a polycyclic aromatic hydrocarbon and the result of incomplete combustion of organic matter at temperatures between and . The ubiquitous compound can be found in coal tar, tobacco smoke and many foods, especially grilled meats. The substance with the formula C20H12 is one of the benzopyrenes, formed by a benzene ring fused to pyrene. Its diol epoxide metabolites (more commonly known as BPDE) react with and bind to DNA, resulting in mutations and eventually cancer. It is listed as a Group 1 carcinogen by the IARC. In the 18th century a scrotal cancer of chimney sweepers, the chimney sweeps' carcinoma, was already known to be connected to soot. Description Benzo 'a''yrene (B''a''P) is a polycyclic aromatic hydrocarbon found in coal tar with the formula C20H12. The compound is one of the benzopyrenes, formed by a benzene ring fused to pyrene, and is the result of incomplete combustion at temperatures between and . Sources The main source ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polycyclic Aromatic Hydrocarbon
A polycyclic aromatic hydrocarbon (PAH) is a class of organic compounds that is composed of multiple aromatic rings. The simplest representative is naphthalene, having two aromatic rings and the three-ring compounds anthracene and phenanthrene. PAHs are uncharged, non-polar and planar. Many are colorless. Many of them are found in coal and in oil deposits, and are also produced by the combustion of organic matter—for example, in engines and incinerators or when biomass burns in forest fires. Polycyclic aromatic hydrocarbons are discussed as possible starting materials for abiotic syntheses of materials required by the earliest forms of life. Nomenclature and structure The terms polyaromatic hydrocarbon or polynuclear aromatic hydrocarbon are also used for this concept. By definition, polycyclic aromatic hydrocarbons have multiple rings, precluding benzene from being considered a PAH. Some sources, such as the US EPA and CDC, consider naphthalene to be the simplest PAH. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |



pyrene_numbered.png)