|
Isotachophoresis
Isotachophoresis (ITP) is a technique in analytical chemistry used for selective separation and concentration of ionic analytes. It is a form of electrophoresis; charged analytes are separated based on ionic mobility, a quantity which tells how fast an ion migrates through an electric field. Overview In conventional ITP separations, a discontinuous buffer system is used. The sample is introduced between a zone of fast leading electrolyte (LE) and a zone of slow terminating (or: trailing) electrolyte (TE). Usually, the LE and the TE have a common counterion, but the co-ions (having charges with the same sign as the analytes of interest) are different: the LE is defined by co-ions with high ionic mobility, while the TE is defined by co-ions with low ionic mobility. The analytes of interest have intermediate ionic mobility. Application of an electric potential results in a low electrical field in the leading electrolyte and a high electrical field in the terminating electrolyte. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Capillary Electrophoresis
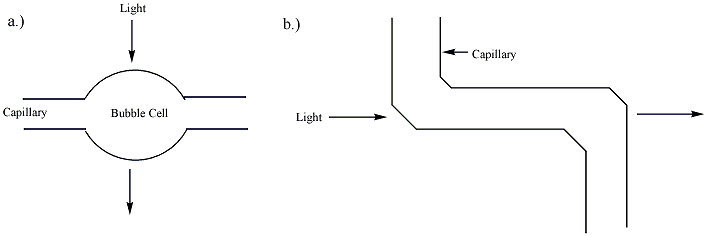
Capillary electrophoresis (CE) is a family of electrokinetic separation methods performed in submillimeter diameter capillaries and in micro- and nanofluidic channels. Very often, CE refers to capillary zone electrophoresis (CZE), but other electrophoretic techniques including capillary gel electrophoresis (CGE), capillary isoelectric focusing (CIEF), capillary isotachophoresis and micellar electrokinetic chromatography (MEKC) belong also to this class of methods. In CE methods, analytes migrate through electrolyte solutions under the influence of an electric field. Analytes can be separated according to ionic mobility and/or partitioning into an alternate phase via non-covalent interactions. Additionally, analytes may be concentrated or "focused" by means of gradients in conductivity and pH. Instrumentation The instrumentation needed to perform capillary electrophoresis is relatively simple. A basic schematic of a capillary electrophoresis system is shown in ''figure 1' ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Capillary Zone Electrophoresis
Capillary electrophoresis (CE) is a family of electrokinetic separation methods performed in submillimeter diameter capillaries and in micro- and nanofluidic channels. Very often, CE refers to capillary zone electrophoresis (CZE), but other electrophoretic techniques including capillary gel electrophoresis (CGE), capillary isoelectric focusing (CIEF), capillary isotachophoresis and micellar electrokinetic chromatography (MEKC) belong also to this class of methods. In CE methods, analytes migrate through electrolyte solutions under the influence of an electric field. Analytes can be separated according to ionic mobility and/or partitioning into an alternate phase via non-covalent interactions. Additionally, analytes may be concentrated or "focused" by means of gradients in conductivity and pH. Instrumentation The instrumentation needed to perform capillary electrophoresis is relatively simple. A basic schematic of a capillary electrophoresis system is shown in ''figure 1'' ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Discontinuous Electrophoresis
Discontinuous electrophoresis (colloquially ''disc electrophoresis'') is a type of polyacrylamide gel electrophoresis. It was developed by Ornstein and Davis. This method produces high resolution and good band definition. It is widely used technique for separating proteins according to size and charge. Method In this method, the gel is divided into two discontinuous parts, resolving and stacking gel, both have different concentrations of polyacrylamide. The one with lower concentration is stacked on top of the one with higher concentration. Discontinuity is based on four parameters: gel structure, pH value of the buffer, ionic strength of the buffer, and the nature of the ions in the gel and electrode buffer. The electrode buffer contains glycine. Glycine has very low net charge at pH 6.8 of stacking gel, so it has low mobility. The proteins are separated according to the principle of isotachophoresis and form stacks in the order of mobility (stacking effect). Mobility depends on ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electrical Mobility
Electrical mobility is the ability of charged particles (such as electrons or protons) to move through a medium in response to an electric field that is pulling them. The separation of ions according to their mobility in gas phase is called ion mobility spectrometry, in liquid phase it is called electrophoresis. Theory When a charged particle in a gas or liquid is acted upon by a uniform electric field, it will be accelerated until it reaches a constant drift velocity according to the formula : v_\text = \mu E, where : v_\text is the drift velocity ( SI units: m/s), : E is the magnitude of the applied electric field (V/m), : \mu is the mobility (m2/(V·s)). In other words, the electrical mobility of the particle is defined as the ratio of the drift velocity to the magnitude of the electric field: : \mu = \frac. For example, the mobility of the sodium ion (Na+) in water at 25 °C is . This means that a sodium ion in an electric field of 1 V/m would have an average d ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dynamic Equilibrium
In chemistry, a dynamic equilibrium exists once a reversible reaction occurs. Substances transition between the reactants and products at equal rates, meaning there is no net change. Reactants and products are formed at such a rate that the concentration of neither changes. It is a particular example of a system in a steady state. In physics, concerning thermodynamics, a closed system is in thermodynamic equilibrium when reactions occur at such rates that the composition of the mixture does not change with time. Reactions do in fact occur, sometimes vigorously, but to such an extent that changes in composition cannot be observed. Equilibrium constants can be expressed in terms of the rate constants for reversible reactions. Examples In a new bottle of soda, the concentration of carbon dioxide in the liquid phase has a particular value. If half of the liquid is poured out and the bottle is sealed, carbon dioxide will leave the liquid phase at an ever-decreasing rate, and th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Normal Distribution
In statistics, a normal distribution or Gaussian distribution is a type of continuous probability distribution for a real-valued random variable. The general form of its probability density function is : f(x) = \frac e^ The parameter \mu is the mean or expectation of the distribution (and also its median and mode), while the parameter \sigma is its standard deviation. The variance of the distribution is \sigma^2. A random variable with a Gaussian distribution is said to be normally distributed, and is called a normal deviate. Normal distributions are important in statistics and are often used in the natural and social sciences to represent real-valued random variables whose distributions are not known. Their importance is partly due to the central limit theorem. It states that, under some conditions, the average of many samples (observations) of a random variable with finite mean and variance is itself a random variable—whose distribution converges to a normal d ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Concentration
In chemistry, concentration is the abundance of a constituent divided by the total volume of a mixture. Several types of mathematical description can be distinguished: '' mass concentration'', ''molar concentration'', '' number concentration'', and '' volume concentration''. The concentration can refer to any kind of chemical mixture, but most frequently refers to solutes and solvents in solutions. The molar (amount) concentration has variants, such as normal concentration and osmotic concentration. Etymology The term concentration comes from the word concentrate, from the French , from con– + center, meaning “to put at the center”. Qualitative description Often in informal, non-technical language, concentration is described in a qualitative way, through the use of adjectives such as "dilute" for solutions of relatively low concentration and "concentrated" for solutions of relatively high concentration. To concentrate a solution, one must add more solute (for exam ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electric Field
An electric field (sometimes E-field) is the physical field that surrounds electrically charged particles and exerts force on all other charged particles in the field, either attracting or repelling them. It also refers to the physical field for a system of charged particles. Electric fields originate from electric charges and time-varying electric currents. Electric fields and magnetic fields are both manifestations of the electromagnetic field, one of the four fundamental interactions (also called forces) of nature. Electric fields are important in many areas of physics, and are exploited in electrical technology. In atomic physics and chemistry, for instance, the electric field is the attractive force holding the atomic nucleus and electrons together in atoms. It is also the force responsible for chemical bonding between atoms that result in molecules. The electric field is defined as a vector field that associates to each point in space the electrostatic ( Coulomb) for ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
ITP Separation Model
ITP may refer to: Companies and organizations * Interactive Theorem Proving (conference), an annual international academic conference * Intertape Polymer Group, a packaging products manufacturer * Institute for Theoretical Physics (other) * Institutes of Technology and Polytechnics, in New Zealand * Independent Theatre Pakistan, a Pakistani theatre company * Industria de Turbo Propulsores, a Spanish gas turbine manufacturer * International Therapeutic Proteins, a company that produces biological therapeutics from polyclonal antibodies * Information Technology & Politics, an organized section of the American Political Science Association with the journal ''Journal of Information Technology & Politics'' * International Third Position, a UK-based political movement * Interurban Transit Partnership, operator of a public transport system in Grand Rapids, Michigan, United States * Islamabad Traffic Police, a traffic police force in Islamabad, Pakistan Computing and tec ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electrolyte
An electrolyte is a medium containing ions that is electrically conducting through the movement of those ions, but not conducting electrons. This includes most soluble salts, acids, and bases dissolved in a polar solvent, such as water. Upon dissolving, the substance separates into cations and anions, which disperse uniformly throughout the solvent. Solid-state electrolytes also exist. In medicine and sometimes in chemistry, the term electrolyte refers to the substance that is dissolved. Electrically, such a solution is neutral. If an electric potential is applied to such a solution, the cations of the solution are drawn to the electrode that has an abundance of electrons, while the anions are drawn to the electrode that has a deficit of electrons. The movement of anions and cations in opposite directions within the solution amounts to a current. Some gases, such as hydrogen chloride (HCl), under conditions of high temperature or low pressure can also function as electrolytes ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electric Field
An electric field (sometimes E-field) is the physical field that surrounds electrically charged particles and exerts force on all other charged particles in the field, either attracting or repelling them. It also refers to the physical field for a system of charged particles. Electric fields originate from electric charges and time-varying electric currents. Electric fields and magnetic fields are both manifestations of the electromagnetic field, one of the four fundamental interactions (also called forces) of nature. Electric fields are important in many areas of physics, and are exploited in electrical technology. In atomic physics and chemistry, for instance, the electric field is the attractive force holding the atomic nucleus and electrons together in atoms. It is also the force responsible for chemical bonding between atoms that result in molecules. The electric field is defined as a vector field that associates to each point in space the electrostatic ( Coulomb) for ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |