|
Induline
Induline is a dye of blue, bluish-red or black shades. Induline consists of a mixture of several intensely colored species, so the name is often indulines. It was one of the first synthetic dyes, discovered in 1863 by J. Dale and Heinrich Caro. The main components of induline are various substituted phenazines. Although induline no longer in use, the related dye nigrosin is still produced commercially. Relationship to other compounds Induline is a derivative of the eurhodines (aminophenazines, aminonaphthophenazines). By means of their diazo derivatives can be de-amidated, yielding in this way azonium salts; consequently they may be considered as amidated azonium salts. The first reaction giving a clue to their constitution was the isolation of the intermediate azophenin by O. Witt, which was proved by Fischer and Hepp to be dianilidoquinone dianil, a similar intermediate compound being found shortly afterwards in the naphthalene series. Azophenin, C30H24N4, is prepared by war ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Heinrich Caro
Heinrich Caro (February 13, 1834 in Posen, Prussia Germany now Poznań, Poland – September 11, 1910 in Dresden), was a German chemist. He was a Sephardic Jew. He started his study of chemistry at the Friedrich Wilhelms University and later chemistry and dyeing in Berlin at the ''Royal Trades Institute''. On the initiative of Nicolaus Druckenmüller, he trained as a calico printer in Germany, worked at Troost's calico printing works in Mülheim and then worked at the chemical firm Roberts, Dale in Manchester. During this time he improved the analysis of madder lake. After he returned to Germany he conducted his military service in 1857 and 1858. He worked in the laboratory of Jacques Meyer the father of Viktor Meyer in Berlin. In 1858 he was able to return to Mühlheim where he was not able to conduct his work. He joined the chemical firm Roberts, Dale in Manchester which he knew from his former visit. During his time in England he improved the extraction of Mauveine from th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
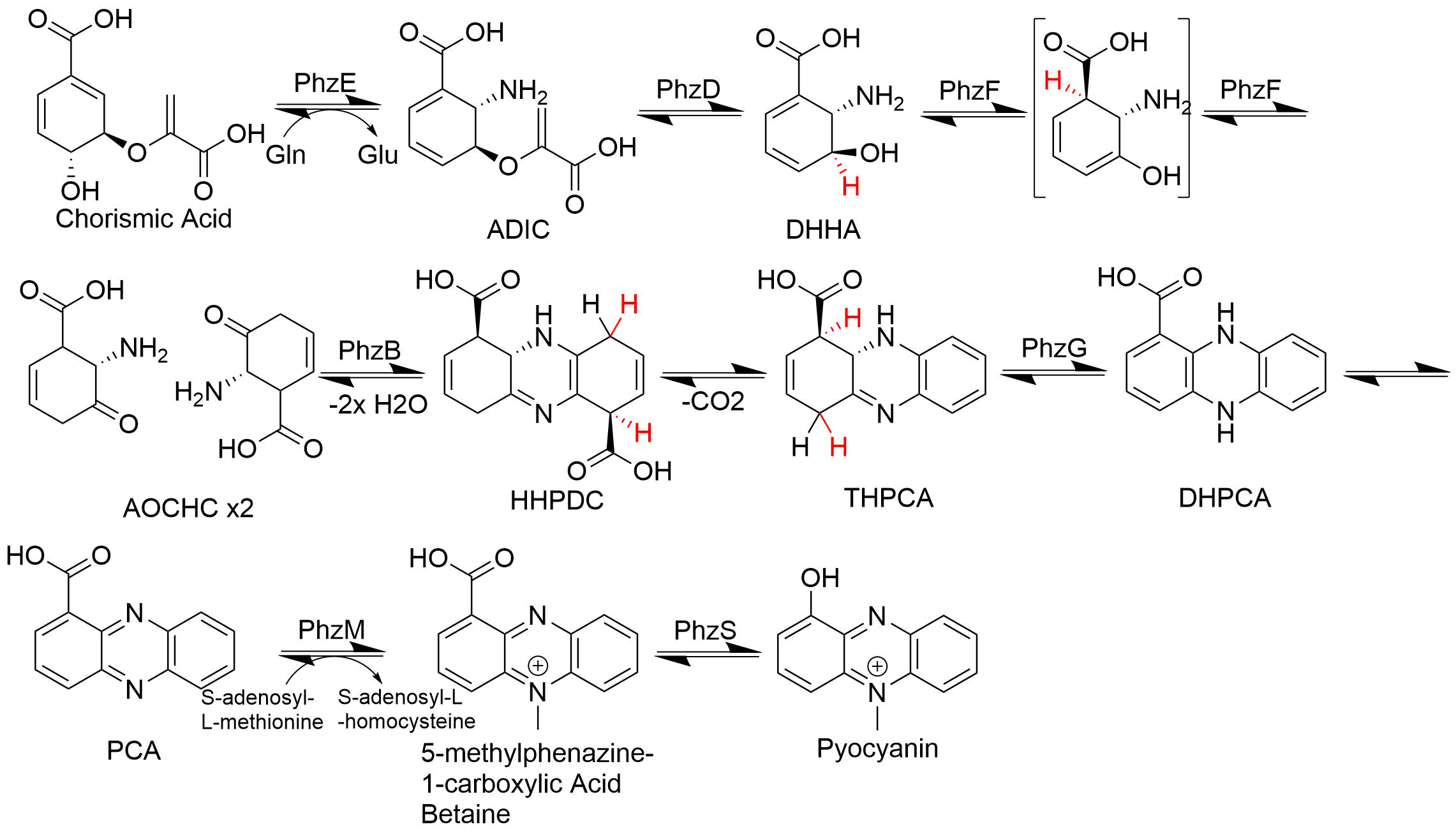
Phenazine
Phenazine is an organic compound with the formula (C6H4)2N2. It is a dibenzo annulated pyrazine, and the parent substance of many dyestuffs, such as the toluylene red, indulines, and safranines (and the closely related eurhodines). Phenazine crystallizes in yellow needles, which are only sparingly soluble in alcohol. Sulfuric acid dissolves it, forming a deep-red solution. Synthesis Classically phenazine are prepared by the reaction of nitrobenzene and aniline in the Wohl-Aue reaction. Other methods include: * pyrolysis of the barium salt of azobenzoate * oxidation of aniline with lead oxide * oxidation of dihydrophenazine, which is prepared by heating pyrocatechin with o-phenylenediamine. * oxidation of ortho-aminodiphenylamine with lead peroxide. Derivatives * The more complex phenazines, such as the naphthophenazines, naphthazines, and naphthotolazines, may be prepared by condensing ortho-diamines with ortho-quinones or by the oxidation of an ortho-diamine in th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nigrosin
In staining dyes, nigrosin (CI 50415, Solvent black 5) is a mixture of black synthetic dyes made by heating a mixture of nitrobenzene, aniline, and hydrochloric acid in the presence of copper or iron. Related to induline, it is a mixture of phenazine-based compounds. Its main industrial uses are as a colorant for lacquers and varnishes and in marker pen inks. Sulfonation of nigrosin yields a water-soluble anionic dye, nigrosin WS (CI 50420, Acid black 2). Uses Aside from its main use in lacquers, small amounts of nigrosin are used for negative staining of bacteria. as well as the capsule-containing fungus ''Cryptococcus neoformans''. The shapes and sizes of the organisms are seen as color-free outlines against the dark background. An advantage of using this method, rather than regular positive stains like methylene blue or carbol fuchsin Carbol fuchsin, carbol-fuchsin, carbolfuchsin, or Castellani's paint (CAS ) is a mixture of phenol and basic fuchsin that is used in bacteria ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Salt (chemistry)
In chemistry, a salt is a chemical compound consisting of an ionic assembly of positively charged cations and negatively charged anions, which results in a compound with no net electric charge. A common example is Salt, table salt, with positively charged sodium ions and negatively charged chloride ions. The component ions in a salt compound can be either inorganic compound, inorganic, such as chloride (Cl−), or organic chemistry, organic, such as acetate (). Each ion can be either monatomic ion, monatomic, such as fluoride (F−), or polyatomic ion, polyatomic, such as sulfate (). Types of salt Salts can be classified in a variety of ways. Salts that produce hydroxide ions when dissolved in water are called ''alkali salts'' and salts that produce hydrogen ions when dissolved in water are called ''acid salts''. ''Neutral salts'' are those salts that are neither acidic nor basic. Zwitterions contain an anionic and a cationic centre in the same molecule, but are not considered s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Naphthalene
Naphthalene is an organic compound with formula . It is the simplest polycyclic aromatic hydrocarbon, and is a white crystalline solid with a characteristic odor that is detectable at concentrations as low as 0.08 ppm by mass. As an aromatic hydrocarbon, naphthalene's structure consists of a fused pair of benzene rings. It is best known as the main ingredient of traditional mothballs. History In the early 1820s, two separate reports described a white solid with a pungent odor derived from the distillation of coal tar. In 1821, John Kidd cited these two disclosures and then described many of this substance's properties and the means of its production. He proposed the name ''naphthaline'', as it had been derived from a kind of naphtha (a broad term encompassing any volatile, flammable liquid hydrocarbon mixture, including coal tar). Naphthalene's chemical formula was determined by Michael Faraday in 1826. The structure of two fused benzene rings was proposed by Emil Erlenm ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aniline
Aniline is an organic compound with the formula C6 H5 NH2. Consisting of a phenyl group attached to an amino group, aniline is the simplest aromatic amine. It is an industrially significant commodity chemical, as well as a versatile starting material for fine chemical synthesis. Its main use is in the manufacture of precursors to polyurethane, dyes, and other industrial chemicals. Like most volatile amines, it has the odor of rotten fish. It ignites readily, burning with a smoky flame characteristic of aromatic compounds. It is toxic to humans. Relative to benzene, it is electron-rich. It thus participates more rapidly in electrophilic aromatic substitution reactions. Likewise, it is also prone to oxidation: while freshly purified aniline is an almost colorless oil, exposure to air results in gradual darkening to yellow or red, due to the formation of strongly colored, oxidized impurities. Aniline can be diazotized to give a diazonium salt, which can then undergo var ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Quinone
The quinones are a class of organic compounds that are formally "derived from aromatic compounds uch as benzene or naphthalene">benzene.html" ;"title="uch as benzene">uch as benzene or naphthalene] by conversion of an even number of –CH= groups into –C(=O)– groups with any necessary rearrangement of double bonds, resulting in "a fully Conjugated system, conjugated cyclic diketone, dione structure". The archetypical member of the class is 1,4-benzoquinone or cyclohexadienedione, often called simply "quinone" (thus the name of the class). Other important examples are 1,2-benzoquinone (''ortho''-quinone), 1,4-naphthoquinone and 9,10-anthraquinone. The name is derived from that of quinic acid (with the suffix "-one" indicating a ketone), since it is one of the compounds obtained upon oxidation of quinic acid. Quinic acid, like quinine is obtained from cinchona bark, called quinaquina in the indigenous languages of Peruvian tribes. Properties Quinones are oxidized derivativ ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrochloride
In chemistry, a hydrochloride is an acid salt resulting, or regarded as resulting, from the reaction of hydrochloric acid with an organic base (e.g. an amine). An alternative name is chlorhydrate, which comes from French. An archaic alternative name is muriate, derived from hydrochloric acid's ancient name: muriatic acid. Uses Converting amines into their hydrochlorides is a common way to improve their water solubility, which can be desirable for substances used in medications. The European Pharmacopoeia lists more than 200 hydrochlorides as active ingredients in medications. These hydrochlorides, compared to free bases, may more readily dissolve in the gastrointestinal tract and be absorbed into the bloodstream more quickly. Additionally, many hydrochlorides of amines have a longer shelf-life than their respective free bases. Amine hydrochlorides represent latent forms of a more reactive free base. In this regard, formation of an amine hydrochloride confers protection. This ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Base (chemistry)
In chemistry, there are three definitions in common use of the word base, known as Arrhenius bases, Brønsted bases, and Lewis bases. All definitions agree that bases are substances that react with acids, as originally proposed by G.-F. Rouelle in the mid-18th century. In 1884, Svante Arrhenius proposed that a base is a substance which dissociates in aqueous solution to form Hydroxide ions OH−. These ions can react with hydrogen ions (H+ according to Arrhenius) from the dissociation of acids to form water in an acid–base reaction. A base was therefore a metal hydroxide such as NaOH or Ca(OH)2. Such aqueous hydroxide solutions were also described by certain characteristic properties. They are slippery to the touch, can taste bitter and change the color of pH indicators (e.g., turn red litmus paper blue). In water, by altering the autoionization equilibrium, bases yield solutions in which the hydrogen ion activity is lower than it is in pure water, i.e., the water ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Salt (chemistry)
In chemistry, a salt is a chemical compound consisting of an ionic assembly of positively charged cations and negatively charged anions, which results in a compound with no net electric charge. A common example is Salt, table salt, with positively charged sodium ions and negatively charged chloride ions. The component ions in a salt compound can be either inorganic compound, inorganic, such as chloride (Cl−), or organic chemistry, organic, such as acetate (). Each ion can be either monatomic ion, monatomic, such as fluoride (F−), or polyatomic ion, polyatomic, such as sulfate (). Types of salt Salts can be classified in a variety of ways. Salts that produce hydroxide ions when dissolved in water are called ''alkali salts'' and salts that produce hydrogen ions when dissolved in water are called ''acid salts''. ''Neutral salts'' are those salts that are neither acidic nor basic. Zwitterions contain an anionic and a cationic centre in the same molecule, but are not considered s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fluorescence
Fluorescence is the emission of light by a substance that has absorbed light or other electromagnetic radiation. It is a form of luminescence. In most cases, the emitted light has a longer wavelength, and therefore a lower photon energy, than the absorbed radiation. A perceptible example of fluorescence occurs when the absorbed radiation is in the ultraviolet region of the electromagnetic spectrum (invisible to the human eye), while the emitted light is in the visible region; this gives the fluorescent substance a distinct color that can only be seen when the substance has been exposed to UV light. Fluorescent materials cease to glow nearly immediately when the radiation source stops, unlike phosphorescent materials, which continue to emit light for some time after. Fluorescence has many practical applications, including mineralogy, gemology, medicine, chemical sensors (fluorescence spectroscopy), fluorescent labelling, dyes, biological detectors, cosmic-ray detection, vac ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |